Leptin is an effective treatment for hypothalamic amenorrhea
- PMID: 21464293
- PMCID: PMC3080974
- DOI: 10.1073/pnas.1015674108
Leptin is an effective treatment for hypothalamic amenorrhea
Abstract
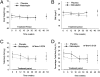
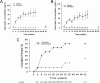
Hypothalamic amenorrhea (HA) is associated with dysfunction of the hypothalamic-pituitary-peripheral endocrine axes, leading to infertility and bone loss, and usually is caused by chronic energy deficiency secondary to strenuous exercise and/or decreased food intake. Energy deficiency also leads to hypoleptinemia, which has been proposed, on the basis of observational studies as well as an open-label study, to mediate the neuroendocrine abnormalities associated with this condition. To prove definitively a causal role of leptin in the pathogenesis of HA, we performed a randomized, double-blinded, placebo-controlled trial of human recombinant leptin (metreleptin) in replacement doses over 36 wk in women with HA. We assessed its effects on reproductive outcomes, neuroendocrine function, and bone metabolism. Leptin replacement resulted in recovery of menstruation and corrected the abnormalities in the gonadal, thyroid, growth hormone, and adrenal axes. We also demonstrated changes in markers of bone metabolism suggestive of bone formation, but no changes in bone mineral density were detected over the short duration of this study. If these data are confirmed, metreleptin administration in replacement doses to normalize circulating leptin levels may prove to be a safe and effective therapy for women with HA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: A study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. - PubMed
-
- Yen SS. Female hypogonadotropic hypogonadism. Hypothalamic amenorrhea syndrome. Endocrinol Metab Clin North Am. 1993;22:29–58. - PubMed
-
- Warren MP, et al. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–877. - PubMed
-
- Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. - PubMed
-
- Misra M, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK058785/DK/NIDDK NIH HHS/United States
- K24 DK081913/DK/NIDDK NIH HHS/United States
- 202579/ERC_/European Research Council/International
- M01-RR-01032/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- R56 DK058785/DK/NIDDK NIH HHS/United States
- GJT08004/TI_/Telethon/Italy
- DK81913/DK/NIDDK NIH HHS/United States
- M01 RR001032/RR/NCRR NIH HHS/United States
- DK58785/DK/NIDDK NIH HHS/United States
- AG032030/AG/NIA NIH HHS/United States
- DK79929/DK/NIDDK NIH HHS/United States
- R01 AG032030/AG/NIA NIH HHS/United States
- R01 DK079929/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

