Ablation of PI3K p110-α prevents high-fat diet-induced liver steatosis
- PMID: 21464441
- PMCID: PMC3292322
- DOI: 10.2337/db10-0869
Ablation of PI3K p110-α prevents high-fat diet-induced liver steatosis
Abstract
Objective: To determine whether the phosphoinositide 3-kinase (PI3K) catalytic subunits p110-α and p110-β play a role in liver steatosis induced by a high-fat diet (HFD).
Research design and methods: Liver-specific p110-α and p110-β knockout mice and control animals for each group were fed an HFD or normal chow for 8 weeks. Biochemical assays and quantitative real-time PCR were used to measure triglyceride, expression of lipogenic and gluconeogenic genes, and activity of protein kinases downstream of PI3K in liver lysates. Fatty acid uptake and incorporation into triglycerides were assessed in isolated hepatocytes.
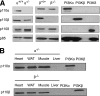
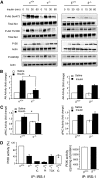
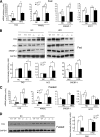
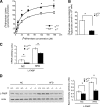
Results: Hepatic triglyceride levels in HFD-fed p110-α(-/-) mice were 84 ± 3% lower than in p110-α(+/+) mice, whereas the loss of p110-β did not significantly alter liver lipid accumulation. p110-α(-/-) livers also showed a reduction in atypical protein kinase C activity and decreased mRNA and protein expression of several lipogenic genes. Hepatocytes isolated from p110-α(-/-) mice exhibited decreased palmitate uptake and reduced fatty acid incorporation into triglycerides as compared with p110-α(+/+) cells, and hepatic expression of liver fatty acid binding protein was lower in p110-α(-/-) mice fed the HFD as compared with controls. Ablation of neither p110-α nor p110-β ameliorated glucose intolerance induced by the HFD, and genes involved in gluconeogenesis were upregulated in the liver of both knockout animals.
Conclusions: PI3K p110-α, and not p110-β, promotes liver steatosis in mice fed an HFD. p110-α might exert this effect in part through activation of atypical protein kinase C, upregulation of lipogenesis, and increased uptake of fatty acids.
Figures

References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–1231 - PubMed
-
- Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2007;25:883–889 - PubMed
-
- Inoue M, Ohtake T, Motomura W, et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 2005;336:215–222 - PubMed
-
- Lin J, Yang R, Tarr PT, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 2005;120:261–273 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

