The Werner syndrome helicase protein is required for cell proliferation, immortalization, and tumorigenesis in Scaffold attachment factor B1 deficient mice
- PMID: 21464516
- PMCID: PMC3091521
- DOI: 10.18632/aging.100300
The Werner syndrome helicase protein is required for cell proliferation, immortalization, and tumorigenesis in Scaffold attachment factor B1 deficient mice
Abstract
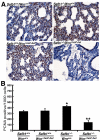
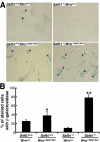
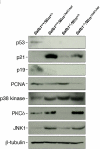
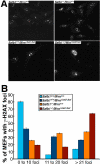
Werner syndrome (WS) is a rare disorder characterized by the premature onset of several pathologies associated with aging. The gene responsible for WS codes for a RecQ-type DNA helicase and is believed to be involved in different aspects of DNA repair, replication, and transcription. We recently identified the Scaffold attachment factor B1 (SAFB1) as a potential interactants in human cells. SAFB1 is a multifunctional protein that binds both nucleic acids and is involved in the attachment of chromatin to the nuclear matrix, transcription, and stress response. Mice lacking SAFB1 exhibit developmental abnormalities in their lungs, high incidence of perinatal lethality, and adults develop different types of tumors. Mouse embryonic fibroblasts from Safb1-null animals are immortalized in culture. In this study, mice with a mutation in the helicase domain of the Wrn gene were crossed to Safb1-null mice. Double homozygous mutant mice exhibited increased apoptosis, a lower cell proliferation rate in their lungs and a higher incidence of perinatal death compared to Safb1-null mice. Few double homozygous mutants survived weaning and died before the age of six months. Finally, mouse embryonic fibroblasts lacking a functional Wrn helicase inhibited the immortalization of Safb1-null cells. These results indicate that an intact Wrn protein is required for immortalization and tumorigenesis in Safb1-null mice.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures



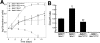

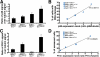
Similar articles
-
Metabolic and Phenotypic Differences between Mice Producing a Werner Syndrome Helicase Mutant Protein and Wrn Null Mice.PLoS One. 2015 Oct 8;10(10):e0140292. doi: 10.1371/journal.pone.0140292. eCollection 2015. PLoS One. 2015. PMID: 26447695 Free PMC article.
-
Divergent cellular phenotypes of human and mouse cells lacking the Werner syndrome RecQ helicase.DNA Repair (Amst). 2010 Jan 2;9(1):11-22. doi: 10.1016/j.dnarep.2009.09.013. Epub 2009 Nov 5. DNA Repair (Amst). 2010. PMID: 19896421 Free PMC article.
-
Werner syndrome protein prevents DNA breaks upon chromatin structure alteration.Aging Cell. 2007 Aug;6(4):471-81. doi: 10.1111/j.1474-9726.2007.00301.x. Epub 2007 May 23. Aging Cell. 2007. PMID: 17521388
-
Telomere ResQue and preservation--roles for the Werner syndrome protein and other RecQ helicases.Mech Ageing Dev. 2008 Jan-Feb;129(1-2):79-90. doi: 10.1016/j.mad.2007.10.007. Epub 2007 Oct 30. Mech Ageing Dev. 2008. PMID: 18054793 Review.
-
Werner syndrome protein: functions in the response to DNA damage and replication stress in S-phase.Exp Gerontol. 2007 Sep;42(9):871-8. doi: 10.1016/j.exger.2007.04.011. Epub 2007 May 10. Exp Gerontol. 2007. PMID: 17587522 Review.
Cited by
-
Targeting an Achilles' heel of cancer with a WRN helicase inhibitor.Cell Cycle. 2013 Oct 15;12(20):3329-35. doi: 10.4161/cc.26320. Epub 2013 Sep 12. Cell Cycle. 2013. PMID: 24036544 Free PMC article.
-
MTOR-driven quasi-programmed aging as a disposable soma theory: blind watchmaker vs. intelligent designer.Cell Cycle. 2013 Jun 15;12(12):1842-7. doi: 10.4161/cc.25062. Epub 2013 Jun 12. Cell Cycle. 2013. PMID: 23708516 Free PMC article.
-
Scaffold attachment factor B2 (SAFB2)-null mice reveal non-redundant functions of SAFB2 compared with its paralog, SAFB1.Dis Model Mech. 2015 Sep;8(9):1121-7. doi: 10.1242/dmm.019885. Epub 2015 Jun 18. Dis Model Mech. 2015. PMID: 26092125 Free PMC article.
References
-
- Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966;45:177–221. - PubMed
-
- Salk D, Au K, Hoehn H, Martin GM. Cytogenetics of Werner's syndrome cultured skin fibroblasts: variegated translocation mosaicism. Cytogenet Cell Genet. 1981;30:92–107. - PubMed
-
- Pagano G, Zatterale A, Degan P, d'Ischia M, Kelly FJ, Pallardo FV, et al. Multiple involvement of oxidative stress in Werner syndrome phenotype. Biogerontology. 2005:233–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

