Structural inferences for Cholera toxin mutations in Vibrio cholerae
- PMID: 21464837
- PMCID: PMC3064844
- DOI: 10.6026/97320630006001
Structural inferences for Cholera toxin mutations in Vibrio cholerae
Abstract
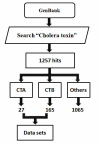
Cholera is a global disease that has persisted for millennia. The cholera toxin (CT) from Vibrio cholerae is responsible for the clinical symptoms of cholera. This toxin is a hetero-hexamer (AB(5)) complex consisting of a subunit A (CTA) with a pentamer (B(5)) of subunit B (CTB). The importance of the AB(5) complex for pathogenesis is established for the wild type O1 serogroup using known structural and functional data. However, its role is not yet documented in other known serogroups harboring sequence level residue mutations. The sequences for the toxin from different serogroups are available in GenBank (release 177). Sequence analysis reveals mutations at several sequence positions in the toxin across serogroups. Therefore, it is of interest to locate the position of these mutations in the AB(5) structure to infer complex assembly for its functional role in different serogroups. We show that mutations in the CTA are at the solvent exposed regions of the AB(5) complex, whereas those in the CTB are at the CTB/CTB interface of the homo-pentamer complex. Thus, the role of mutations at the CTB/CTB interface for B(5) complex assembly is implied. It is observed that these mutations are often non-synonymous (e.g. polar to non-polar or vice versa). The formation of the AB(5) complex involves inter-subunit residue-residue interactions at the protein-protein interfaces. Hence, these mutations, at the structurally relevant positions, are of importance for the understanding of pathogenesis by several serogroups. This is also of significance in the improvement of recombinant CT protein complex analogs for vaccine design and their use against multiple serogroups.
Keywords: Cholera toxin (CT); O1/O139; Vibrio cholerae; mutation; non O1/O139; protein-protein interfaces.
Figures





Mapping of CTA mutations to CTA/CTB interface residues in CTA (Please refer to Figure 1 for the visual illustration of CTA/CTB interface).
Mapping of CTB mutations to CTB (D subunit)/CTB (E subunit) interface residues (Please refer to Figure 5 for the visual illustration of D-E interface).
Mapping of CTB mutations to CTB (D subunit)/CTB (H subunit) interface residues (Please refer to Figure 5 for the visual illustration of D-H interface).
A total of 6 unique mutations thus observed among the known CTA sequences (Table 2 in supplementary material) from several serogroups are shown at their corresponding 6 residue positions using the Corey-Pauling-Kultun (CPK) residue model representation.
Fourteen unique mutations thus observed among the known CTB sequences (Table 3 in supplementary material) from several serogroups are shown at their corresponding 13 residue positions using the CPK residue model representation.

A total of 6 unique mutations thus observed among the known CTA sequences (Table 4 in supplementary material) from several serogroups are shown at their corresponding 6 residue positions using the CPK residue model representation. All of these 6 mutated positions are present at the solvent exposed regions of CTA in both monomer and CTA/CTB complex state.
A total of 7 out of 14 unique mutations thus observed among the known CTB sequences (Table 4 in supplementary material) from several serogroups are shown at their corresponding 7 (3, 15, 25, 34, 47,52 and 60) out of the 13 residue positions using the CPK residue model representation are at the CTB/CTB interfaces in the B5 complex.
References
LinkOut - more resources
Full Text Sources
