The forest behind the tree: phylogenetic exploration of a dominant Mycobacterium tuberculosis strain lineage from a high tuberculosis burden country
- PMID: 21464915
- PMCID: PMC3064675
- DOI: 10.1371/journal.pone.0018256
The forest behind the tree: phylogenetic exploration of a dominant Mycobacterium tuberculosis strain lineage from a high tuberculosis burden country
Abstract
Background: Genotyping of Mycobacterium tuberculosis isolates is a powerful tool for epidemiological control of tuberculosis (TB) and phylogenetic exploration of the pathogen. Standardized PCR-based typing, based on 15 to 24 mycobacterial interspersed repetitive unit-variable number of tandem repeat (MIRU-VNTR) loci combined with spoligotyping, has been shown to have adequate resolution power for tracing TB transmission and to be useful for predicting diverse strain lineages in European settings. Its informative value needs to be tested in high TB-burden countries, where the use of genotyping is often complicated by dominance of geographically specific, genetically homogeneous strain lineages.
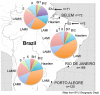
Methodology/principal findings: We tested this genotyping system for molecular epidemiological analysis of 369 M. tuberculosis isolates from 3 regions of Brazil, a high TB-burden country. Deligotyping, targeting 43 large sequence polymorphisms (LSPs), and the MIRU-VNTRplus identification database were used to assess phylogenetic predictions. High congruence between the different typing results consistently revealed the countrywide supremacy of the Latin-American-Mediterranean (LAM) lineage, comprised of three main branches. In addition to an already known RDRio branch, at least one other branch characterized by a phylogenetically informative LAM3 spoligo-signature seems to be globally distributed beyond Brazil. Nevertheless, by distinguishing 321 genotypes in this strain population, combined MIRU-VNTR typing and spoligotyping demonstrated the presence of multiple distinct clones. The use of 15 to 24 loci discriminated 21 to 25% more strains within the LAM lineage, compared to a restricted lineage-specific locus set suggested to be used after SNP analysis. Noteworthy, 23 of the 28 molecular clusters identified were exclusively composed of patient isolates from a same region, consistent with expected patterns of mostly local TB transmission.
Conclusions/significance: Standard MIRU-VNTR typing combined with spoligotyping can reveal epidemiologically meaningful clonal diversity behind a dominant M. tuberculosis strain lineage in a high TB-burden country and is useful to explore international phylogenetical ramifications.
Conflict of interest statement
Figures



References
-
- Organization WH. Geneva: W.H.O; 2006. Global Tuberculosis Control, WHO Report.
-
- Allix-Beguec C, Fauville-Dufaux M, Stoffels K, Ommeslag D, Walravens K, et al. Importance of identifying Mycobacterium bovis as a causative agent of human tuberculosis. Eur Respir J. 2010;35:692–694. - PubMed
-
- Barnes PF, Cave MD. Molecular epidemiology of tuberculosis. N Engl J Med. 2003;349:1149–1156. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

