Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA
- PMID: 21464946
- PMCID: PMC3064590
- DOI: 10.1371/journal.pone.0017942
Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA
Abstract
Background: In Plasmodium falciparum malaria endemic areas placental malaria (PM) is an important complication of malaria. The recurrence of malaria in primigravidae women irrespective of acquired protection during childhood is caused by the interaction between the parasite-expressed VAR2CSA antigen and chondroitin sulfate A (CSA) in the placental intervillous space and lack of protective antibodies. PM impairs fetal development mainly by excessive inflammation processes. After infections during pregnancy women acquire immunity to PM conferred by antibodies against VAR2CSA. Ideally, a vaccine against PM will induce antibody-mediated immune responses that block the adhesion of infected erythrocytes (IE) in the placenta.
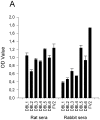
Principal findings: We have previously shown that antibodies raised in rat against individual domains of VAR2CSA can block IE binding to CSA. In this study we have immunized mice, rats and rabbits with each individual domain and the full-length protein corresponding to the FCR3 VAR2CSA variant. We found there is an inherently higher immunogenicity of C-terminal domains compared to N-terminally located domains. This was irrespective of whether antibodies were induced against single domains or the full-length protein. Species-specific antibody responses were also found, these were mainly directed against single domains and not the full-length VAR2CSA protein.
Conclusions/significance: Binding inhibitory antibodies appeared to be against conformational B-cell epitopes. Non-binding inhibitory antibodies reacted highly against the C-terminal end of the VAR2CSA molecule especially the highly polymorphic DBL6ε domain. Differential species-specific induction of antibody responses may allow for more direct analysis of functional versus non-functional B-cell epitopes.
Conflict of interest statement
Figures
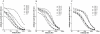
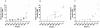
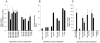

Similar articles
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
-
VAR2CSA binding phenotype has ancient origin and arose before Plasmodium falciparum crossed to humans: implications in placental malaria vaccine design.Sci Rep. 2019 Nov 18;9(1):16978. doi: 10.1038/s41598-019-53334-8. Sci Rep. 2019. PMID: 31740695 Free PMC article.
-
A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates.Elife. 2022 Feb 1;11:e76264. doi: 10.7554/eLife.76264. Elife. 2022. PMID: 35103596 Free PMC article.
-
Identification of Id1-DBL2X of VAR2CSA as a key domain inducing highly inhibitory and cross-reactive antibodies.Vaccine. 2012 Feb 8;30(7):1343-8. doi: 10.1016/j.vaccine.2011.12.065. Epub 2012 Jan 5. Vaccine. 2012. PMID: 22226864
-
Progress and Insights Toward an Effective Placental Malaria Vaccine.Front Immunol. 2021 Feb 25;12:634508. doi: 10.3389/fimmu.2021.634508. eCollection 2021. Front Immunol. 2021. PMID: 33717176 Free PMC article. Review.
Cited by
-
First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria.Clin Infect Dis. 2019 Oct 15;69(9):1509-1516. doi: 10.1093/cid/ciy1140. Clin Infect Dis. 2019. PMID: 30629148 Free PMC article. Clinical Trial.
-
Identification and characterization of B-cell epitopes in the DBL4ε domain of VAR2CSA.PLoS One. 2012;7(9):e43663. doi: 10.1371/journal.pone.0043663. Epub 2012 Sep 6. PLoS One. 2012. PMID: 22970138 Free PMC article.
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
-
Prospects and Pitfalls of Pregnancy-Associated Malaria Vaccination Based on the Natural Immune Response to Plasmodium falciparum VAR2CSA-Expressing Parasites.Malar Res Treat. 2011;2011:764845. doi: 10.4061/2011/764845. Epub 2012 Jan 18. Malar Res Treat. 2011. PMID: 22363896 Free PMC article.
-
Antigen reversal identifies targets of opsonizing IgGs against pregnancy-associated malaria.Infect Immun. 2014 Nov;82(11):4842-53. doi: 10.1128/IAI.02097-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156731 Free PMC article.
References
-
- Aagaard C, Dietrich J, Doherty M, Andersen P. TB vaccines: current status and future perspectives. Immunol Cell Biol. 2009;87:279–286. - PubMed
-
- Guinovart C, Alonso PL. Methods for determining vaccine efficacy and effectiveness and the main barriers to developing a fully deployable malaria vaccine. Am J Trop Med Hyg. 2007;77:276–281. - PubMed
-
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

