Mitotic defects lead to pervasive aneuploidy and accompany loss of RB1 activity in mouse LmnaDhe dermal fibroblasts
- PMID: 21464947
- PMCID: PMC3064591
- DOI: 10.1371/journal.pone.0018065
Mitotic defects lead to pervasive aneuploidy and accompany loss of RB1 activity in mouse LmnaDhe dermal fibroblasts
Abstract
Background: Lamin A (LMNA) is a component of the nuclear lamina and is mutated in several human diseases, including Emery-Dreifuss muscular dystrophy (EDMD; OMIM ID# 181350) and the premature aging syndrome Hutchinson-Gilford progeria syndrome (HGPS; OMIM ID# 176670). Cells from progeria patients exhibit cell cycle defects in both interphase and mitosis. Mouse models with loss of LMNA function have reduced Retinoblastoma protein (RB1) activity, leading to aberrant cell cycle control in interphase, but how mitosis is affected by LMNA is not well understood.
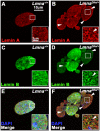
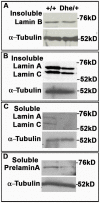
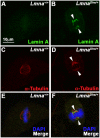
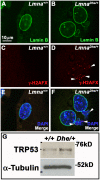
Results: We examined the cell cycle and structural phenotypes of cells from mice with the Lmna allele, Disheveled hair and ears (Lmna(Dhe)). We found that dermal fibroblasts from heterozygous Lmna(Dhe) (Lmna(Dhe/+)) mice exhibit many phenotypes of human laminopathy cells. These include severe perturbations to the nuclear shape and lamina, increased DNA damage, and slow growth rates due to mitotic delay. Interestingly, Lmna(Dhe/+) fibroblasts also had reduced levels of hypophosphorylated RB1 and the non-SMC condensin II-subunit D3 (NCAP-D3), a mitosis specific centromere condensin subunit that depends on RB1 activity. Mitotic check point control by mitotic arrest deficient-like 1 (MAD2L1) also was perturbed in Lmna(Dhe/+) cells. Lmna(Dhe/+) fibroblasts were consistently aneuploid and had higher levels of micronuclei and anaphase bridges than normal fibroblasts, consistent with chromosome segregation defects.
Conclusions: These data indicate that RB1 may be a key regulator of cellular phenotype in laminopathy-related cells, and suggest that the effects of LMNA on RB1 include both interphase and mitotic cell cycle control.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

