Gdnf is mitogenic, neurotrophic, and chemoattractive to enteric neural crest cells in the embryonic colon
- PMID: 21465624
- PMCID: PMC3092856
- DOI: 10.1002/dvdy.22630
Gdnf is mitogenic, neurotrophic, and chemoattractive to enteric neural crest cells in the embryonic colon
Abstract
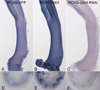
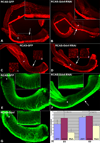
Glial-derived neurotrophic factor (Gdnf) is required for morphogenesis of the enteric nervous system (ENS) and it has been shown to regulate proliferation, differentiation, and survival of cultured enteric neural crest-derived cells (ENCCs). The goal of this study was to investigate its in vivo role in the colon, the site most commonly affected by intestinal neuropathies such as Hirschsprung's disease. Gdnf activity was modulated in ovo in the distal gut of avian embryos using targeted retrovirus-mediated gene overexpression and retroviral vector-based gene silencing. We find that Gdnf has a pleiotropic effect on colonic ENCCs, promoting proliferation, inducing neuronal differentiation, and acting as a chemoattractant. Down-regulating Gdnf similarly induces premature neuronal differentiation, but also inhibits ENCC proliferation, leading to distal colorectal aganglionosis with severe proximal hypoganglionosis. These results indicate an important role for Gdnf signaling in colonic ENS formation and emphasize the critical balance between proliferation and differentiation in the developing ENS.
Copyright © 2011 Wiley-Liss, Inc.
Figures



Similar articles
-
Enhancement of enteric neural stem cell neurogenesis by glial cell-derived neurotrophic factor in experimental Hirschsprung's disease.Pediatr Surg Int. 2024 Oct 26;40(1):274. doi: 10.1007/s00383-024-05861-3. Pediatr Surg Int. 2024. PMID: 39460767
-
Regulators of gene expression in Enteric Neural Crest Cells are putative Hirschsprung disease genes.Dev Biol. 2016 Aug 1;416(1):255-265. doi: 10.1016/j.ydbio.2016.06.004. Epub 2016 Jun 4. Dev Biol. 2016. PMID: 27266404
-
Immunophenotypic characterization of enteric neural crest cells in the developing avian colorectum.Dev Dyn. 2012 May;241(5):842-51. doi: 10.1002/dvdy.23767. Epub 2012 Mar 23. Dev Dyn. 2012. PMID: 22411589 Free PMC article.
-
Neuron-Glia Interaction in the Developing and Adult Enteric Nervous System.Cells. 2020 Dec 31;10(1):47. doi: 10.3390/cells10010047. Cells. 2020. PMID: 33396231 Free PMC article. Review.
-
Roles of Enteric Neural Stem Cell Niche and Enteric Nervous System Development in Hirschsprung Disease.Int J Mol Sci. 2021 Sep 7;22(18):9659. doi: 10.3390/ijms22189659. Int J Mol Sci. 2021. PMID: 34575824 Free PMC article. Review.
Cited by
-
A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease.J Clin Invest. 2015 Dec;125(12):4483-96. doi: 10.1172/JCI83178. Epub 2015 Nov 16. J Clin Invest. 2015. PMID: 26571399 Free PMC article. Clinical Trial.
-
The road best traveled: Neural crest migration upon the extracellular matrix.Semin Cell Dev Biol. 2020 Apr;100:177-185. doi: 10.1016/j.semcdb.2019.10.013. Epub 2019 Nov 11. Semin Cell Dev Biol. 2020. PMID: 31727473 Free PMC article. Review.
-
[Effect of enhancer of zeste homolog 2 on the expression of glial cell line-derived neurotrophic factor family receptor α-1 in the colon tissue of children with Hirschsprung's disease].Zhongguo Dang Dai Er Ke Za Zhi. 2019 Oct;21(10):1033-1037. doi: 10.7499/j.issn.1008-8830.2019.10.015. Zhongguo Dang Dai Er Ke Za Zhi. 2019. PMID: 31642440 Free PMC article. Chinese.
-
A distinct transcriptome characterizes neural crest-derived cells at the migratory wavefront during enteric nervous system development.Development. 2023 Mar 1;150(5):dev201090. doi: 10.1242/dev.201090. Epub 2023 Mar 6. Development. 2023. PMID: 36779913 Free PMC article.
-
PleiotRHOpic: Rho pathways are essential for all stages of Neural Crest development.Small GTPases. 2014;5:e27975. doi: 10.4161/sgtp.27975. Epub 2014 Mar 10. Small GTPases. 2014. PMID: 24614304 Free PMC article. Review.
References
-
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. - PubMed
-
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. - PubMed
-
- Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. - PubMed
-
- Cepko CL. Transduction of genes using retroviral vectors. In: Ausubel RB FM, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1991. pp. 9:9.10.11–19.14.13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

