Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools
- PMID: 21466232
- PMCID: PMC3095683
- DOI: 10.1021/tx200031q
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools
Abstract
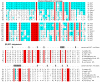
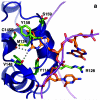
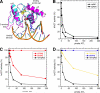
O(6)-Alkylguanine-DNA alkyltransferase (AGT) is a widely distributed, unique DNA repair protein that acts as a single agent to directly remove alkyl groups located on the O(6)-position of guanine from DNA restoring the DNA in one step. The protein acts only once, and its alkylated form is degraded rapidly. It is a major factor in counteracting the mutagenic, carcinogenic, and cytotoxic effects of agents that form such adducts including N-nitroso-compounds and a number of cancer chemotherapeutics. This review describes the structure, function, and mechanism of action of AGTs and of a family of related alkyltransferase-like proteins, which do not act alone to repair O(6)-alkylguanines in DNA but link repair to other pathways. The paradoxical ability of AGTs to stimulate the DNA-damaging ability of dihaloalkanes and other bis-electrophiles via the formation of AGT-DNA cross-links is also described. Other important properties of AGTs include the ability to provide resistance to cancer therapeutic alkylating agents, and the availability of AGT inhibitors such as O(6)-benzylguanine that might overcome this resistance is discussed. Finally, the properties of fusion proteins in which AGT sequences are linked to other proteins are outlined. Such proteins occur naturally, and synthetic variants engineered to react specifically with derivatives of O(6)-benzylguanine are the basis of a valuable research technique for tagging proteins with specific reagents.
Figures






References
-
- Pegg AE, Dolan ME, Moschel RC. Structure, function and inhibition of O6-alkylguanine-DNA alkyltransferase. Progr. Nucleic Acids Res. Mol. Biol. 1995;51:167–223. - PubMed
-
- Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat. Res. 2000;462:83–100. - PubMed
-
- Margison G, Povey AC, Kaina B, Santibáñez-Koref MF. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. - PubMed
-
- Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer. 2004;4:296–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

