An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells
- PMID: 21466612
- PMCID: PMC11159804
- DOI: 10.1111/j.1349-7006.2011.01948.x
An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells
Abstract
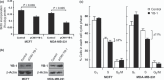
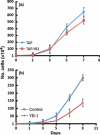
The Y-box binding protein 1 (YB-1) is a multifunctional protein that affects transcription, splicing, and translation. Overexpression of YB-1 in breast cancers causes cisplatin resistance. The exact mechanism by which YB-1 confers cisplatin resistance is unknown. The aim of the present study was to identify, using mass spectrometry, proteins that interact with YB-1 that are important for cisplatin resistance in two breast cancer cell lines, namely MCF7 and MDA-MB-231. A tagged YB-1 construct was used to identify proteins interacting directly with YB-1 in breast cancer cells. We then focused on proteins that are potentially involved in breast cancer progression based on the ONCOMINE public microarray database. Genes encoding for these YB-1-interacting proteins were examined in the public NCBI comparative genomic hybridization database to determine whether they are localized to regions of chromosomes that are rearranged in breast cancer tissues. From these analyses, we generated a list of proteins potentially involved in cisplatin resistance. Cisplatin dose-response curves were constructed in MCF7 and MDA-MB-231 transfected with four siRNA corresponding to each of these YB-1 interactors to identify proteins significantly affecting cisplatin sensitivity upon gene silencing. Depletion of only the X-linked ribosomal protein S4 (RPS4X) resulted in consistent resistance to cisplatin in both cell lines with at least three different siRNA sequences against RPS4X. Further analyses indicated that the knock down of RPS4X decreased DNA synthesis, induced cisplatin resistance, and is equivalent to the overexpression of YB-1 in both MCF7 and MDA-MB-231 cells. These results suggest that the RPS4X/YB-1 complex is a significant potential target to counteract cisplatin resistance in breast cancer.
© 2011 Japanese Cancer Association.
Figures




Similar articles
-
NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines.Mol Cancer. 2011 Nov 25;10:145. doi: 10.1186/1476-4598-10-145. Mol Cancer. 2011. PMID: 22118625 Free PMC article.
-
The strand separation and nuclease activities associated with YB-1 are dispensable for cisplatin resistance but overexpression of YB-1 in MCF7 and MDA-MB-231 breast tumor cells generates several chemoresistance signatures.Int J Biochem Cell Biol. 2008;40(11):2492-507. doi: 10.1016/j.biocel.2008.04.011. Epub 2008 May 15. Int J Biochem Cell Biol. 2008. PMID: 18571458
-
The human endonuclease III enzyme is a relevant target to potentiate cisplatin cytotoxicity in Y-box-binding protein-1 overexpressing tumor cells.Cancer Sci. 2008 Apr;99(4):762-9. doi: 10.1111/j.1349-7006.2008.00739.x. Epub 2008 Feb 27. Cancer Sci. 2008. PMID: 18307537 Free PMC article.
-
[Molecular mechanism of the stress induction of MDR1 gene].Nihon Rinsho. 1997 May;55(5):1054-8. Nihon Rinsho. 1997. PMID: 9155152 Review. Japanese.
-
Cisplatin resistance and transcription factors.Curr Med Chem Anticancer Agents. 2005 Jan;5(1):15-27. doi: 10.2174/1568011053352587. Curr Med Chem Anticancer Agents. 2005. PMID: 15720258 Review.
Cited by
-
An uncertainty-based interpretable deep learning framework for predicting breast cancer outcome.BMC Bioinformatics. 2024 Feb 29;25(1):88. doi: 10.1186/s12859-024-05716-7. BMC Bioinformatics. 2024. PMID: 38418940 Free PMC article.
-
NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines.Mol Cancer. 2011 Nov 25;10:145. doi: 10.1186/1476-4598-10-145. Mol Cancer. 2011. PMID: 22118625 Free PMC article.
-
Phospho-ΔNp63α/microRNA feedback regulation in squamous carcinoma cells upon cisplatin exposure.Cell Cycle. 2013 Feb 15;12(4):684-97. doi: 10.4161/cc.23598. Epub 2013 Jan 23. Cell Cycle. 2013. PMID: 23343772 Free PMC article.
-
Chemotherapy Resistance: Role of Mitochondrial and Autophagic Components.Cancers (Basel). 2022 Mar 12;14(6):1462. doi: 10.3390/cancers14061462. Cancers (Basel). 2022. PMID: 35326612 Free PMC article. Review.
-
Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging.Brain Sci. 2023 Jan 24;13(2):195. doi: 10.3390/brainsci13020195. Brain Sci. 2023. PMID: 36831738 Free PMC article. Review.
References
-
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res 2001; 478: 23–43. - PubMed
-
- Janz M, Harbeck N, Dettmar P et al. Y‐box factor YB‐1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI‐1. Int J Cancer 2002; 97: 278–82. - PubMed
-
- Swamynathan SK, Nambiar A, Guntaka RV. Role of single‐stranded DNA regions and Y‐box proteins in transcriptional regulation of viral and cellular genes. FASEB J 1998; 12: 515–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

