A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time
- PMID: 21466676
- PMCID: PMC3219386
- DOI: 10.1186/cc10129
A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time
Abstract
Introduction: Fresh frozen plasma (FFP) is widely used, but few studies have described patterns of plasma use in critical care. We carried out a multicentre study of coagulopathy in intensive care units (ICUs) and here describe overall FFP utilisation in adult critical care, the indications for transfusions, factors indicating the doses used and the effects of FFP use on coagulation.
Methods: We conducted a prospective, multicentre, observational study of all patients sequentially admitted to 29 adult UK general ICUs over 8 weeks. Daily data throughout ICU admission were collected concerning coagulation, relevant clinical outcomes (including bleeding), coagulopathy (defined as international normalised ratio (INR) >1.5, or equivalent prothrombin time (PT)), FFP and cryoprecipitate use and indications for transfusion.
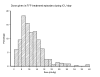
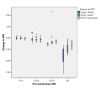
Results: Of 1,923 admissions, 12.7% received FFP in the ICU during 404 FFP treatment episodes (1,212 FFP units). Overall, 0.63 FFP units/ICU admission were transfused (0.11 units/ICU day). Reasons for FFP transfusion were bleeding (48%), preprocedural prophylaxis (15%) and prophylaxis without planned procedure (36%). Overall, the median FFP dose was 10.8 ml kg⁻¹, but doses varied widely (first to third quartile, 7.2 to 14.4 ml kg⁻¹). Thirty-one percent of FFP treatments were to patients without PT prolongation, and 41% were to patients without recorded bleeding and only mildly deranged INR (<2.5). Higher volumes of FFP were administered when the indication was bleeding (median doses: bleeding 11.1 ml kg⁻¹, preprocedural prophylaxis 9.8 ml kg⁻¹, prophylaxis without procedure 8.9 ml kg⁻¹; P = 0.009 across groups) and when the pretransfusion INR was higher (ranging from median dose 8.9 ml kg⁻¹ at INR ≤ 1.5 to 15.7 ml kg⁻¹ at INR >3; P < 0.001 across ranges). Regression analyses suggested bleeding was the strongest predictor of higher FFP dose. Pretransfusion INR was more frequently normal when the transfusion indication was bleeding. Overall, posttransfusion corrections of INR were consistently small unless the pretransfusion INR was >2.5, but administration during bleeding was associated with greater INR corrections.
Conclusions: There is wide variation in FFP use by ICU clinicians, and a high proportion of current FFP transfusions are of unproven clinical benefit. Better evidence from clinical trials could significantly alter patterns of use and modify current treatment costs.
Figures
Comment in
-
The conundrum of persistent inappropriate use of frozen plasma.Crit Care. 2011;15(3):160. doi: 10.1186/cc10215. Epub 2011 May 25. Crit Care. 2011. PMID: 21635704 Free PMC article.
References
-
- Palo R, Capraro L, Hovilehto S, Koivuranta M, Krusius T, Loponen E, Mäntykoski R, Pentti J, Pitkänen O, Raitakari M, Rimpiläinen J, Salmenperä M, Salo H, Mäki T. Population-based audit of fresh frozen plasma transfusion practices. Transfusion. 2006;46:1921–1925. doi: 10.1111/j.1537-2995.2006.00998.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

