Comparative cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human and sperm whale (Physeter macrocephalus) skin cells
- PMID: 21466859
- PMCID: PMC4084666
- DOI: 10.1016/j.cbpc.2011.03.011
Comparative cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human and sperm whale (Physeter macrocephalus) skin cells
Abstract
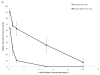
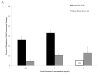
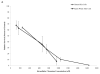
Chromium (Cr) is a global marine pollutant, present in marine mammal tissues. Hexavalent chromium [Cr(VI)] is a known human carcinogen. In this study, we compare the cytotoxic and clastogenic effects of Cr(VI) in human (Homo sapiens) and sperm whale (Physeter macrocephalus) skin fibroblasts. Our data show that increasing concentrations of both particulate and soluble Cr(VI) induce increasing amounts of cytotoxicity and clastogenicity in human and sperm whale skin cells. Furthermore, the data show that sperm whale cells are resistant to these effects exhibiting less cytotoxicity and genotoxicity than the human cells. Differences in Cr uptake accounted for some but not all of the differences in particulate and soluble Cr(VI) genotoxicity, although it did explain the differences in particulate Cr(VI) cytotoxicity. Altogether, the data indicate that Cr(VI) is a genotoxic threat to whales, but also suggest that whales have evolved cellular mechanisms to protect them against the genotoxicity of environmental agents such as Cr(VI).
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures











Similar articles
-
Cytotoxicity and genotoxicity of hexavalent chromium in human and North Atlantic right whale (Eubalaena glacialis) lung cells.Comp Biochem Physiol C Toxicol Pharmacol. 2009 Nov;150(4):487-94. doi: 10.1016/j.cbpc.2009.07.004. Epub 2009 Jul 24. Comp Biochem Physiol C Toxicol Pharmacol. 2009. PMID: 19632355 Free PMC article.
-
Prolonged exposure to particulate Cr(VI) is cytotoxic and genotoxic to fin whale cells.J Trace Elem Med Biol. 2020 Dec;62:126562. doi: 10.1016/j.jtemb.2020.126562. Epub 2020 May 26. J Trace Elem Med Biol. 2020. PMID: 32570008 Free PMC article.
-
The genotoxicity of particulate and soluble chromate in sperm whale (physeter macrocephalus) skin fibroblasts.Environ Mol Mutagen. 2011 Jan;52(1):43-9. doi: 10.1002/em.20579. Environ Mol Mutagen. 2011. PMID: 20839228 Free PMC article.
-
Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms.Mutat Res. 2003 Dec 10;533(1-2):3-36. doi: 10.1016/j.mrfmmm.2003.09.006. Mutat Res. 2003. PMID: 14643411 Review.
-
Chromium (III) and chromium (VI) as important players in the induction of genotoxicity - current view.Ann Agric Environ Med. 2021 Mar 18;28(1):1-10. doi: 10.26444/aaem/118228. Epub 2020 Mar 10. Ann Agric Environ Med. 2021. PMID: 33775062 Review.
Cited by
-
A comparison of particulate hexavalent chromium cytotoxicity and genotoxicity in human and leatherback sea turtle lung cells from a one environmental health perspective.Toxicol Appl Pharmacol. 2019 Aug 1;376:70-81. doi: 10.1016/j.taap.2019.05.013. Epub 2019 May 18. Toxicol Appl Pharmacol. 2019. PMID: 31108106 Free PMC article.
-
Hexavalent chromium is cytotoxic and genotoxic to hawksbill sea turtle cells.Toxicol Appl Pharmacol. 2014 Sep 1;279(2):113-8. doi: 10.1016/j.taap.2014.06.008. Epub 2014 Jun 19. Toxicol Appl Pharmacol. 2014. PMID: 24952338 Free PMC article.
-
A three year study of metal levels in skin biopsies of whales in the Gulf of Mexico after the Deepwater Horizon oil crisis.Comp Biochem Physiol C Toxicol Pharmacol. 2018 Feb;205:15-25. doi: 10.1016/j.cbpc.2017.12.003. Epub 2017 Dec 20. Comp Biochem Physiol C Toxicol Pharmacol. 2018. PMID: 29277452 Free PMC article.
-
Comparative cytotoxicity and genotoxicity of soluble and particulate hexavalent chromium in human and hawksbill sea turtle (Eretmochelys imbricata) skin cells.Comp Biochem Physiol C Toxicol Pharmacol. 2015 Dec;178:145-155. doi: 10.1016/j.cbpc.2015.09.013. Epub 2015 Oct 9. Comp Biochem Physiol C Toxicol Pharmacol. 2015. PMID: 26440299 Free PMC article.
-
Role of DNA methylation in cell cycle arrest induced by Cr (VI) in two cell lines.PLoS One. 2013 Aug 6;8(8):e71031. doi: 10.1371/journal.pone.0071031. Print 2013. PLoS One. 2013. PMID: 23940686 Free PMC article.
References
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Chromium. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2008.
-
- Bodannes RS, Chan PC. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979;105:195–196. - PubMed
-
- Geisler CD, Schmidt D. An overview of chromium in the marine environment. Dt. Hydrogr. Z. 1991;44:185–196.
-
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2005;203:167–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

