Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein
- PMID: 21467030
- PMCID: PMC3103305
- DOI: 10.1074/jbc.M110.200311
Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein
Abstract
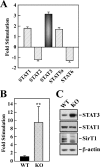
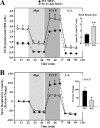
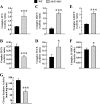
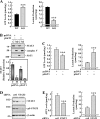
In mammals, the transcriptional activity of signal transducer and activator of transcription 3 (STAT3) is regulated by the deacetylase SIRT1. However, whether the newly described nongenomic actions of STAT3 toward mitochondrial oxidative phosphorylation are dependent on SIRT1 is unclear. In this study, Sirt1 gene knock-out murine embryonic fibroblast (MEF) cells were used to delineate the role of SIRT1 in the regulation of STAT3 mitochondrial function. Here, we show that STAT3 mRNA and protein levels and the accumulation of serine-phosphorylated STAT3 in mitochondria were increased significantly in Sirt1-KO cells as compared with wild-type MEFs. Various mitochondrial bioenergetic parameters, such as the oxygen consumption rate in cell cultures, enzyme activities of the electron transport chain complexes in isolated mitochondria, and production of ATP and lactate, indicated that Sirt1-KO cells exhibited higher mitochondrial respiration as compared with wild-type MEFs. Two independent approaches, including ectopic expression of SIRT1 and siRNA-mediated knockdown of STAT3, led to reduction in intracellular ATP levels and increased lactate production in Sirt1-KO cells that were approaching those of wild-type controls. Comparison of profiles of phospho-antibody array data indicated that the deletion of SirT1 was accompanied by constitutive activation of the pro-inflammatory NF-κB pathway, which is key for STAT3 induction and increased cellular respiration in Sirt1-KO cells. Thus, SIRT1 appears to be a functional regulator of NF-κB-dependent STAT3 expression that induces mitochondrial biogenesis. These results have implications for understanding the interplay between STAT3 and SIRT1 in pro-inflammatory conditions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

