Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon
- PMID: 21467034
- PMCID: PMC3121453
- DOI: 10.1074/jbc.M111.223693
Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon
Abstract
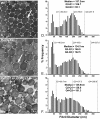
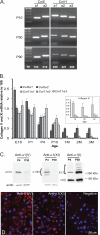
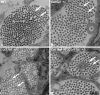
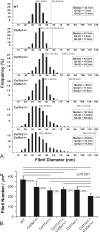
Collagens V and XI comprise a single regulatory type of fibril-forming collagen with multiple isoforms. Both co-assemble with collagen I or II to form heterotypic fibrils and have been implicated in regulation of fibril assembly. The objective of this study was to determine the roles of collagens V and XI in the regulation of tendon fibrillogenesis. Flexor digitorum longus tendons from a haplo-insufficient collagen V mouse model of classic Ehlers Danlos syndrome (EDS) had decreased biomechanical stiffness compared with controls consistent with joint laxity in EDS patients. However, fibril structure was relatively normal, an unexpected finding given the altered fibrils observed in dermis and cornea from this model. This suggested roles for other related molecules, i.e. collagen XI, and compound Col5a1(+/-),Col11a1(+/-) tendons had altered fibril structures, supporting a role for collagen XI. To further evaluate this, transcript expression was analyzed in wild type tendons. During development (E18-P10) both collagen V and XI were comparably expressed; however, collagen V predominated in mature (P30) tendons. The collagens had a similar expression pattern. Tendons with altered collagen V and/or XI expression (Col5a1(+/-); Col11a1(+/-); Col5a1(+/-),Col11a1(+/-); Col11a1(-/-); Col5a1(+/-),Col11a1(-/-)) were analyzed at E18. All genotypes demonstrated a reduced fibril number and altered structure. This phenotype was more severe with a reduction in collagen XI. However, the absence of collagen XI with a reduction in collagen V was associated with the most severe fibril phenotype. The data demonstrate coordinate roles for collagens V and XI in the regulation of fibril nucleation and assembly during tendon development.
Figures
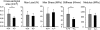

References
-
- Beighton P. (1992) in McKusick's Heritable Disorders of Connective Tissue (Beighton P. ed) pp. 189–251, Mosby, St. Louis, MO
-
- Steinmann B. (2002) in Connective Tissue and Its Heritable Disorders (Royce P. ed) pp. 431–523, Wiley-Liss, Inc., New York, NY
-
- Sokolov B. P., Prytkov A. N., Tromp G., Knowlton R. G., Prockop D. J. (1991) Hum. Genet. 88, 125–129 - PubMed
-
- Wordsworth B. P., Ogilvie D. J., Sykes B. C. (1991) Br. J. Rheumatol. 30, 173–177 - PubMed
-
- Burrows N. P., Nicholls A. C., Yates J. R., Gatward G., Sarathachandra P., Richards A., Pope F. M. (1996) J. Invest. Dermatol. 106, 1273–1276 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

