Kinetics of activated thrombin-activatable fibrinolysis inhibitor (TAFIa)-catalyzed cleavage of C-terminal lysine residues of fibrin degradation products and removal of plasminogen-binding sites
- PMID: 21467042
- PMCID: PMC3103306
- DOI: 10.1074/jbc.M110.215061
Kinetics of activated thrombin-activatable fibrinolysis inhibitor (TAFIa)-catalyzed cleavage of C-terminal lysine residues of fibrin degradation products and removal of plasminogen-binding sites
Abstract
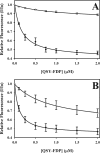
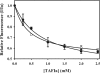
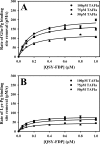
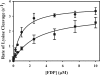
Partial digestion of fibrin by plasmin exposes C-terminal lysine residues, which comprise new binding sites for both plasminogen and tissue-type plasminogen activator (tPA). This binding increases the catalytic efficiency of plasminogen activation by 3000-fold compared with tPA alone. The activated thrombin-activatable fibrinolysis inhibitor (TAFIa) attenuates fibrinolysis by removing these residues, which causes a 97% reduction in tPA catalytic efficiency. The aim of this study was to determine the kinetics of TAFIa-catalyzed lysine cleavage from fibrin degradation products and the kinetics of loss of plasminogen-binding sites. We show that the k(cat) and K(m) of Glu(1)-plasminogen (Glu-Pg)-binding site removal are 2.34 s(-1) and 142.6 nm, respectively, implying a catalytic efficiency of 16.21 μm(-1) s(-1). The corresponding values of Lys(77)/Lys(78)-plasminogen (Lys-Pg)-binding site removal are 0.89 s(-1) and 96 nm implying a catalytic efficiency of 9.23 μm(-1) s(-1). These catalytic efficiencies of plasminogen-binding site removal by TAFIa are the highest of any TAFIa-catalyzed reaction with a biological substrate reported to date and suggest that plasmin-modified fibrin is a primary physiological substrate for TAFIa. We also show that the catalytic efficiency of cleavage of all C-terminal lysine residues, whether they are involved in plasminogen binding or not, is 1.10 μm(-1) s(-1). Interestingly, this value increases to 3.85 μm(-1) s(-1) in the presence of Glu-Pg. These changes are due to a decrease in K(m). This suggests that an interaction between TAFIa and plasminogen comprises a component of the reaction mechanism, the plausibility of which was established by showing that TAFIa binds both Glu-Pg and Lys-Pg.
Figures

References
-
- Collen D. (1999) Thromb. Haemost. 82, 259–270 - PubMed
-
- Fredenburgh J. C., Nesheim M. E. (1992) J. Biol. Chem. 267, 26150–26156 - PubMed
-
- Horrevoets A. J., Pannekoek H., Nesheim M. E. (1997) J. Biol. Chem. 272, 2176–2182 - PubMed
-
- Bajzar L., Manuel R., Nesheim M. E. (1995) J. Biol. Chem. 270, 14477–14484 - PubMed
-
- Mao S. S., Cooper C. M., Wood T., Shafer J. A., Gardell S. J. (1999) J. Biol. Chem. 274, 35046–35052 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

