Reporting of eligibility criteria of randomised trials: cohort study comparing trial protocols with subsequent articles
- PMID: 21467104
- PMCID: PMC3071036
- DOI: 10.1136/bmj.d1828
Reporting of eligibility criteria of randomised trials: cohort study comparing trial protocols with subsequent articles
Abstract
Objective: To determine whether and how eligibility criteria of participants prespecified in protocols of randomised trials are reported in subsequent articles.
Design: Cohort study.
Setting: Protocols submitted to the ethics committee of a German medical faculty.
Data sources: 52 trial protocols and 78 subsequent publications published between 2000 and 2006.
Main outcome measure: Proportion of matching, missing, modified, or newly added eligibility criteria between trial protocols and subsequent publications.
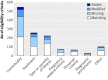
Results: Differences were found between protocols and subsequent publications for all 52 trials. Information on eligibility criteria was missing in the publications for all 52 trials (100%, 95% confidence interval 93% to 100%), modified for 44 (85%, 72% to 93%), and newly added for 21 (41%, 27% to 55%). The mean number of eligibility criteria for each trial was 25 (range 7-43) and the mean proportion of matching eligibility criteria per trial was 50% (95% confidence interval 44% to 55%, range 13-93). Of 1248 eligibility criteria prespecified in the protocols, 606 (49%, 46% to 51%) were matching in subsequent publications, 479 (38%, 36% to 41%) were missing, and 163 (13%, 11% to 15%) were modified. 51 eligibility criteria were added to publications. Most prespecified eligibility criteria were about comorbidity (42%, 39% to 45%), treatment (20%, 18% to 22%), or type or severity of illness (17%, 15% to 19%). Most of the missing eligibility criteria (96%, 94% to 97%) and modified eligibility criteria (54%, 46% to 62%) suggested broader study populations and most of the added eligibility criteria (86%, 74% to 94%) suggested narrower study populations.
Conclusions: Many users of trial information rely on published journal articles. These articles generally do not reflect the exact definition of the study population as prespecified in the protocol. Incomplete or inadequate reporting of eligibility criteria hampers a proper assessment of the applicability of trial results.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Similar articles
-
Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials.J Natl Cancer Inst. 2016 May 25;108(11). doi: 10.1093/jnci/djw129. Print 2016 Nov. J Natl Cancer Inst. 2016. PMID: 27226519
-
Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications.BMJ. 2014 Jul 16;349:g4539. doi: 10.1136/bmj.g4539. BMJ. 2014. PMID: 25030633 Free PMC article.
-
Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols.AIDS. 2005 Nov 4;19(16):1885-96. doi: 10.1097/01.aids.0000189866.67182.f7. AIDS. 2005. PMID: 16227797
-
Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study.BMJ. 2005 May 7;330(7499):1049. doi: 10.1136/bmj.38414.422650.8F. Epub 2005 Apr 7. BMJ. 2005. PMID: 15817527 Free PMC article. Review.
-
Evolution in the eligibility criteria of randomized controlled trials for systemic cancer therapies.Cancer Treat Rev. 2016 Feb;43:67-73. doi: 10.1016/j.ctrv.2015.12.006. Epub 2015 Dec 31. Cancer Treat Rev. 2016. PMID: 26827694 Review.
Cited by
-
Characteristics of randomised trials on diseases in the digestive system registered in ClinicalTrials.gov: a retrospective analysis.BMJ Open. 2011 Jan 1;1(2):e000309. doi: 10.1136/bmjopen-2011-000309. BMJ Open. 2011. PMID: 22080540 Free PMC article.
-
Comparison of reporting phase I trial results in ClinicalTrials.gov and matched publications.Invest New Drugs. 2017 Dec;35(6):827-833. doi: 10.1007/s10637-017-0510-8. Epub 2017 Sep 14. Invest New Drugs. 2017. PMID: 28905282 Review.
-
Protecting intellectual property associated with Canadian academic clinical trials--approaches and impact.Trials. 2012 Dec 27;13:243. doi: 10.1186/1745-6215-13-243. Trials. 2012. PMID: 23270486 Free PMC article.
-
Review and publication of protocol submissions to Trials - what have we learned in 10 years?Trials. 2016 Dec 16;18(1):34. doi: 10.1186/s13063-016-1743-0. Trials. 2016. PMID: 28114958 Free PMC article.
-
CONSORT 2025 explanation and elaboration: updated guideline for reporting randomised trials.BMJ. 2025 Apr 14;389:e081124. doi: 10.1136/bmj-2024-081124. BMJ. 2025. PMID: 40228832 Free PMC article.
References
-
- Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med 2009;6:e1000144. - PMC - PubMed
-
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. - PubMed
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources