Discriminative stimulus effects of tramadol in humans
- PMID: 21467190
- PMCID: PMC3126638
- DOI: 10.1124/jpet.111.181131
Discriminative stimulus effects of tramadol in humans
Abstract
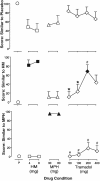
Tramadol is an unscheduled atypical analgesic that acts as an agonist at μ-opioid receptors and inhibits monoamine reuptake. Tramadol can suppress opioid withdrawal, and chronic administration can produce opioid physical dependence; however, diversion and abuse of tramadol is low. The present study further characterized tramadol in a three-choice discrimination procedure. Nondependent volunteers with active stimulant and opioid use (n = 8) participated in this residential laboratory study. Subjects were trained to discriminate between placebo, hydromorphone (8 mg), and methylphenidate (60 mg), and tests of acquisition confirmed that all volunteers could discriminate between the training drugs. The following drug conditions were then tested during discrimination test sessions: placebo, hydromorphone (4 and 8 mg), methylphenidate (30 and 60 mg), and tramadol (50, 100, 200, and 400 mg). In addition to discrimination measures, which included discrete choice, point distribution, and operant responding, subjective and physiological effects were measured for each test condition. Both doses of hydromorphone and methylphenidate were identified as hydromorphone- and methylphenidate-like, respectively. Lower doses of tramadol were generally identified as placebo, with higher doses (200 and 400 mg) identified as hydromorphone, or opioid-like. The highest dose of tramadol increased ratings on the stimulant scale, but was not significantly identified as methylphenidate-like. Tramadol did not significantly increase subjective ratings associated with reinforcement. Taken together, these results extend previous work with tramadol as a potential medication for the treatment of opioid dependence and withdrawal, showing acute doses of tramadol exhibit a profile of effects similar to opioid agonists and may have abuse liability in certain populations.
Figures

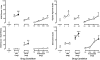
References
-
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, Senay EC, Woody GE. (2006) A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage 31:465–476 - PubMed
-
- Bickel WK, Bigelow GE, Preston KL, Liebson IA. (1989) Opioid drug discrimination in humans: stability, specificity and relation to self-reported drug effect. J Pharmacol Exp Ther 251:1053–1063 - PubMed
-
- Cami J, Lama X, Farre M. (1994) Acute effects of tramadol in methadone-maintained volunteers. Drugs 47 (Suppl 1):39–43 - PubMed
-
- Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL. (2006) Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol 14:109–120 - PubMed
-
- Cicero TJ, Adams EH, Geller A, Inciardi JA, Muñoz A, Schnoll SH, Senay EC, Woody GE. (1999) A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 57:7–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

