Uterine cysts in female mice deficient for caveolin-1 and insulin-like 3 receptor RXFP2
- PMID: 21467199
- PMCID: PMC3100621
- DOI: 10.1210/en.2010-1015
Uterine cysts in female mice deficient for caveolin-1 and insulin-like 3 receptor RXFP2
Abstract
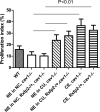
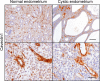
Gene mutations of insulin-like 3 (INSL3) peptide or its G protein-coupled receptor RXFP2 (relaxin family peptide receptor 2) lead to cryptorchidism. The role of INSL3 in adult females is less known, although INSL3 expression has been described in female reproductive organs. Caveolin-1 (CAV1), the main component of caveoli cell membrane invaginations, has been shown to play an important role in epithelial organization and stromal-epithelial interactions. We created a null allele of Cav1 mice by deleting its second exon through embryonic stem cell targeting. Immunohistochemical analysis demonstrated that CAV1 expression was primarily localized to endothelial blood vessel cells and the myometrium uterus, whereas the strongest expression of Rxfp2 was detected in the endometrial epithelium. By 12 months of age approximately 18% of Cav1-/- females developed single or multiple dilated endometrial cysts lined by a flattened, simple low epithelium. A deficiency for Rxfp2 on Cav1-deficient background led to more than a 2-fold increase in the incidence of uterine cysts (54-58%). Appearance of cysts led to a severe disorganization of uterine morphology. We have found that the cysts had an increased expression of β-catenin and estrogen receptor β in endometrial stromal and epithelial cells and increased epithelial proliferation. An analysis of simple dilated cysts in human patients for CAV1 expression did not show appreciable differences with control regardless of menstrual phase, suggesting an involvement of additional factors in human disease. The results of this study suggest a novel synergistic role of INSL3/RXFP2 and CAV1 in structural maintenance of the uterus.
Figures



Similar articles
-
Suppression of insulin-like3 receptor reveals the role of β-catenin and Notch signaling in gubernaculum development.Mol Endocrinol. 2011 Jan;25(1):170-83. doi: 10.1210/me.2010-0330. Epub 2010 Dec 8. Mol Endocrinol. 2011. PMID: 21147849 Free PMC article.
-
Insulin-like 3 signaling is important for testicular descent but dispensable for spermatogenesis and germ cell survival in adult mice.Biol Reprod. 2012 Dec 21;87(6):143. doi: 10.1095/biolreprod.112.103382. Print 2012 Jun. Biol Reprod. 2012. PMID: 23100620 Free PMC article.
-
The insulin-like factor 3 (INSL3)-receptor (RXFP2) network functions as a germ cell survival/anti-apoptotic factor in boar testes.Endocrinology. 2015 Apr;156(4):1523-39. doi: 10.1210/en.2014-1473. Epub 2015 Jan 6. Endocrinology. 2015. PMID: 25562614
-
INSL3/RXFP2 signaling in testicular descent.Ann N Y Acad Sci. 2009 Apr;1160:197-204. doi: 10.1111/j.1749-6632.2009.03841.x. Ann N Y Acad Sci. 2009. PMID: 19416188 Free PMC article. Review.
-
Insulin-like peptide 3 (INSL3) is a major regulator of female reproductive physiology.Hum Reprod Update. 2018 Nov 1;24(6):639-651. doi: 10.1093/humupd/dmy029. Hum Reprod Update. 2018. PMID: 30204868 Review.
Cited by
-
Evidence for existence of insulin-like factor 3 (INSL3) hormone-receptor system in the ovarian corpus luteum and extra-ovarian reproductive organs during pregnancy in goats.Cell Tissue Res. 2021 Jul;385(1):173-189. doi: 10.1007/s00441-021-03410-1. Epub 2021 Feb 15. Cell Tissue Res. 2021. PMID: 33590284
-
Insulin-like peptide 3 in domestic animals with normal and abnormal reproductive functions, in comparison to rodents and humans.Reprod Med Biol. 2022 Sep 22;21(1):e12485. doi: 10.1002/rmb2.12485. eCollection 2022 Jan-Dec. Reprod Med Biol. 2022. PMID: 36310659 Free PMC article. Review.
-
Diverse functions of insulin-like 3 peptide.J Endocrinol. 2020 Oct 1;247(1):R1-R12. doi: 10.1530/JOE-20-0168. J Endocrinol. 2020. PMID: 32813485 Free PMC article. Review.
-
Targeting the relaxin/insulin-like family peptide receptor 1 and 2 with small molecule compounds.Mol Cell Endocrinol. 2019 May 1;487:40-44. doi: 10.1016/j.mce.2018.12.013. Epub 2018 Dec 24. Mol Cell Endocrinol. 2019. PMID: 30590098 Free PMC article. Review.
-
Caveolin-1 Regulation and Function in Mouse Uterus during Early Pregnancy and under Human In Vitro Decidualization.Int J Mol Sci. 2022 Mar 28;23(7):3699. doi: 10.3390/ijms23073699. Int J Mol Sci. 2022. PMID: 35409055 Free PMC article.
References
-
- Bogatcheva NV, Agoulnik AI. 2005. INSL3/LGR8 role in testicular descent and cryptorchidism. Reprod Biomed Online 10:49–54 - PubMed
-
- Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI. 2003. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol 17:2639–2646 - PubMed
-
- Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. 2002. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem 277:31283–31286 - PubMed
-
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. 1999. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13:681–691 - PubMed
-
- Nef S, Parada LF. 1999. Cryptorchidism in mice mutant for Insl3. Nat Genet 22:295–299 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

