Complex regulation of GH autofeedback under dual-peptide drive: studies under a pharmacological GH and sex steroid clamp
- PMID: 21467302
- PMCID: PMC3118586
- DOI: 10.1152/ajpendo.00054.2011
Complex regulation of GH autofeedback under dual-peptide drive: studies under a pharmacological GH and sex steroid clamp
Abstract
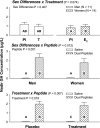
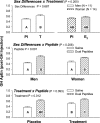
To test the postulate that sex difference, sex steroids, and peptidyl secretagogues control GH autofeedback, 11 healthy postmenopausal women and 14 older men were each given 1) a single iv pulse of GH to enforce negative feedback and 2) continuous iv infusion of saline vs. combined GHRH/GHRP-2 to drive feedback escape during pharmacological estradiol (E(2); women) or testosterone (T; men) supplementation vs. placebo in a double-blind, prospectively randomized crossover design. By three-way ANCOVA, sex difference, sex hormone treatment, peptide stimulation, and placebo/saline responses (covariate) controlled total (integrated) GH recovery during feedback (each P < 0.001). Both sex steroid milieu (P = 0.019) and dual-peptide stimulation (P < 0.001) determined nadir (maximally feedback-suppressed) GH concentrations. E(2)/T exposure elevated nadir GH concentrations during saline infusion (P = 0.003), whereas dual-peptide infusion did so independently of T/E(2) and sex difference (P = 0.001). All three of sex difference (P = 0.001), sex steroid treatment (P = 0.005), and double-peptide stimulation (P < 0.001) augmented recovery of peak (maximally feedback-escaped) GH concentrations. Peak GH responses to dual-peptidyl agonists were greater in women than in men (P = 0.016). E(2)/T augmented peak GH recovery during saline infusion (P < 0.001). Approximate entropy analysis corroborated independent effects of sex steroid treatment (P = 0.012) and peptide infusion (P < 0.001) on GH regularity. In summary, sex difference, sex steroid supplementation, and combined peptide drive influence nadir, peak, and entropic measurements of GH release under controlled negative feedback. To the degree that the pharmacological sex steroid, GH, and dual-peptide clamps provide prephysiological regulatory insights, these outcomes suggest major determinants of pulsatile GH secretion in the feedback domain.
Figures





Similar articles
-
Gender, sex-steroid, and secretagogue-selective recovery from growth hormone-induced feedback in older women and men.J Clin Endocrinol Metab. 2011 Aug;96(8):2540-7. doi: 10.1210/jc.2011-0298. Epub 2011 May 25. J Clin Endocrinol Metab. 2011. PMID: 21613353 Free PMC article. Clinical Trial.
-
Impact of estradiol supplementation on dual peptidyl drive of GH secretion in postmenopausal women.J Clin Endocrinol Metab. 2002 Feb;87(2):859-66. doi: 10.1210/jcem.87.2.8251. J Clin Endocrinol Metab. 2002. PMID: 11836333 Clinical Trial.
-
E2 supplementation selectively relieves GH's autonegative feedback on GH-releasing peptide-2-stimulated GH secretion.J Clin Endocrinol Metab. 2001 Dec;86(12):5904-11. doi: 10.1210/jcem.86.12.8076. J Clin Endocrinol Metab. 2001. PMID: 11739462 Clinical Trial.
-
Sex-steroid modulation of growth hormone (GH) secretory control: three-peptide ensemble regulation under dual feedback restraint by GH and IGF-I.Endocrine. 2003 Oct;22(1):25-40. doi: 10.1385/ENDO:22:1:25. Endocrine. 2003. PMID: 14610296 Review.
-
Neurophysiological regulation and target-tissue impact of the pulsatile mode of growth hormone secretion in the human.Growth Horm IGF Res. 2001 Jun;11 Suppl A:S25-37. doi: 10.1016/s1096-6374(01)80005-8. Growth Horm IGF Res. 2001. PMID: 11527085 Review.
Cited by
-
Gender determines ACTH recovery from hypercortisolemia in healthy older humans.Metabolism. 2013 Dec;62(12):1819-29. doi: 10.1016/j.metabol.2013.08.014. Epub 2013 Sep 25. Metabolism. 2013. PMID: 24074810 Free PMC article. Clinical Trial.
-
Hormone therapy for sexual function in perimenopausal and postmenopausal women.Cochrane Database Syst Rev. 2023 Aug 24;8(8):CD009672. doi: 10.1002/14651858.CD009672.pub3. Cochrane Database Syst Rev. 2023. PMID: 37619252 Free PMC article. Review.
-
Regulated recovery of pulsatile growth hormone secretion from negative feedback: a preclinical investigation.Am J Physiol Regul Integr Comp Physiol. 2011 Oct;301(4):R1143-52. doi: 10.1152/ajpregu.00293.2011. Epub 2011 Jul 27. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21795635 Free PMC article. Clinical Trial.
-
Network identification of hormonal regulation.PLoS One. 2014 May 22;9(5):e96284. doi: 10.1371/journal.pone.0096284. eCollection 2014. PLoS One. 2014. PMID: 24852517 Free PMC article.
References
-
- Aguila MC, Boggaram V, McCann SM. Insulin-like growth factor-I modulates hypothalamic somatostatin through growth hormone releasing-factor increased somatostatin release and messenger ribonucleic acid levels. Brain Res 625: 213–218, 1993 - PubMed
-
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 86: 551–560, 2001 - PubMed
-
- Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Broglio F, Deghenghi R, Ghigo E. Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRPs in human aging. Pituitary 1: 51–58, 1998 - PubMed
-
- Barinaga M, Bilezikjian LM, Vale WW, Rosenfeld MG, Evans RM. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature 314: 279–281, 1985 - PubMed
-
- Bowers CY. Synergistic release of growth hormone by GHRP and GHRH: scope and implication. In: Growth Hormone Secretagogues in Clinical Practice, edited by Bercu BB, Walker RF. New York: Marcel Dekker, 1998, p. 1–25
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

