Prolactin-mediated regulation of lipid biosynthesis genes in vivo in the lactating mammary epithelial cell
- PMID: 21467304
- PMCID: PMC3118595
- DOI: 10.1152/ajpendo.00083.2011
Prolactin-mediated regulation of lipid biosynthesis genes in vivo in the lactating mammary epithelial cell
Abstract
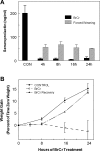
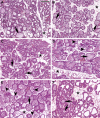
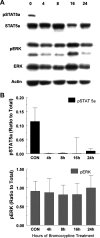
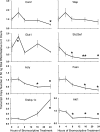
Prolactin (PRL) is known to play an essential role in mammary alveolar proliferation in the pregnant mouse, but its role in lactation has been more difficult to define. Genetic manipulations that alter expression of the PRL receptor and its downstream signaling molecules resulted in developmental defects that may directly or indirectly impact secretory activation and lactation. To examine the in vivo role of PRL specifically in lactation, bromocriptine (BrCr) was administered every 8 h to lactating mice on the second day postpartum, resulting in an ~95% decrease in serum PRL levels. Although morphological changes in secretory alveoli were slight, by 8 h of BrCr, pup growth was inhibited significantly. Phosphorylated STAT5 fell to undetectable levels within 4 h. Decreased milk protein gene expression, β-casein, and α-lactalbumin, was observed after 8 h of treatment. To assess mammary-specific effects on lipid synthesis genes, we isolated mammary epithelial cells (MECs) depleted of mammary adipocytes. Expression of genes involved in glucose uptake, glycolysis, pentose phosphate shunt, de novo synthesis of fatty acids, and biosynthesis of triacylglycerides was decreased up to 19-fold in MECs by just 8 h of BrCr treatment. Glands from BrCr-treated mice showed a twofold reduction in intracellular cytoplasmic lipid droplets and a reduction in cytosolic β-casein. These data demonstrate that PRL signaling regulates MEC-specific lipogenic gene expression and that PRL signals coordinate the milk synthesis and mammary epithelial cell survival during lactation in the mouse.
Figures


Similar articles
-
Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with β-casein expression in mammary epithelial cells.Biochim Biophys Acta. 2016 Aug;1863(8):2006-16. doi: 10.1016/j.bbamcr.2016.04.023. Epub 2016 Apr 26. Biochim Biophys Acta. 2016. PMID: 27130254
-
Integration of lipid metabolism in the mammary gland and adipose tissue by prolactin during lactation.Mol Cell Biochem. 1990 Mar 27;93(2):185-94. doi: 10.1007/BF00226191. Mol Cell Biochem. 1990. PMID: 2345543
-
Prolactin regulates LAT1 expression via STAT5 (signal transducer and activator of transcription 5) signaling in mammary epithelial cells of dairy cows.J Dairy Sci. 2020 Jul;103(7):6627-6634. doi: 10.3168/jds.2019-17945. Epub 2020 May 7. J Dairy Sci. 2020. PMID: 32389479
-
New insights into the importance of prolactin in dairy ruminants.J Dairy Sci. 2016 Jan;99(1):864-74. doi: 10.3168/jds.2015-10035. Epub 2015 Nov 5. J Dairy Sci. 2016. PMID: 26547648 Review.
-
Effects of short day photoperiod on prolactin signaling in dry cows: a common mechanism among tissues and environments?J Anim Sci. 2008 Mar;86(13 Suppl):10-4. doi: 10.2527/jas.2007-0311. Epub 2007 Aug 8. J Anim Sci. 2008. PMID: 17686892 Review.
Cited by
-
Constitutive expression of microRNA-150 in mammary epithelium suppresses secretory activation and impairs de novo lipogenesis.Development. 2016 Nov 15;143(22):4236-4248. doi: 10.1242/dev.139642. Epub 2016 Oct 11. Development. 2016. PMID: 27729410 Free PMC article.
-
Polymorphisms of the PRLR Gene and Their Association with Milk Production Traits in Egyptian Buffaloes.Animals (Basel). 2021 Apr 25;11(5):1237. doi: 10.3390/ani11051237. Animals (Basel). 2021. PMID: 33923003 Free PMC article.
-
Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation.Am J Physiol Endocrinol Metab. 2013 Sep 15;305(6):E700-16. doi: 10.1152/ajpendo.00052.2013. Epub 2013 Jul 23. Am J Physiol Endocrinol Metab. 2013. PMID: 23880316 Free PMC article.
-
Parental factors that impact the ecology of human mammary development, milk secretion, and milk composition-a report from "Breastmilk Ecology: Genesis of Infant Nutrition (BEGIN)" Working Group 1.Am J Clin Nutr. 2023 Apr;117 Suppl 1(Suppl 1):S11-S27. doi: 10.1016/j.ajcnut.2022.11.026. Am J Clin Nutr. 2023. PMID: 37173058 Free PMC article.
-
Impact of Maternal Obesity on the Metabolism and Bioavailability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding.Nutrients. 2020 Dec 23;13(1):19. doi: 10.3390/nu13010019. Nutrients. 2020. PMID: 33374585 Free PMC article. Review.
References
-
- Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J Biol Chem 278: 35988–35999, 2003 - PubMed
-
- Bernichtein S, Kinet S, Jeay S, Llovera M, Madern D, Martial JA, Kelly PA, Goffin V. S179D-human PRL, a pseudophosphorylated human PRL analog, is an agonist and not an antagonist. Endocrinology 142: 3950–3963, 2001 - PubMed
-
- Choi KM, Barash I, Rhoads RE. Insulin and prolactin synergistically stimulate beta-casein messenger ribonucleic acid translation by cytoplasmic polyadenylation. Mol Endocrinol 18: 1670–1686, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

