ALK1 as an emerging target for antiangiogenic therapy of cancer
- PMID: 21467543
- PMCID: PMC3143549
- DOI: 10.1182/blood-2011-01-330142
ALK1 as an emerging target for antiangiogenic therapy of cancer
Abstract
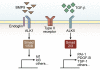
Members of the TGF-β family act on many, if not all, cell types within the body, producing diverse and complex cellular outcomes. Activation of the endothelial cell-restricted TGF-β type I receptor ALK1 results from the binding of several different ligands of the TGF-β family, including bone morphogenetic protein (BMP) 9, BMP10, and TGF-β. Mounting genetic, pharmacologic, and histopathologic evidence supports a critical role for ALK1 signaling in regulation of both developmental and pathologic blood vessel formation. However, the precise function of TGF-β family signaling in endothelial cells is difficult to predict and appears highly context dependent because of the multitude of ligands and receptors influencing the final outcome. Pharmacologic inhibitors of ALK1 have recently been developed and will allow for more accurate studies of ALK1 function in vivo, as well as for assessment of ALK1 as a target for suppression of angiogenesis during tumor development. Herein, we will summarize the current view of ALK1 regulation of endothelial cell phenotype in vitro and in vivo as well as provide an outlook for the ongoing clinical trials of ALK1 inhibitors in malignant disease.
Figures


References
-
- ten Dijke P, Ichijo H, Franzén P, et al. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993;8:2879–2887. - PubMed
-
- Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. - PubMed
-
- Schmierer B, Hill CS. TGFbeta-smad signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. - PubMed
-
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of theTGF-beta receptor. Nature. 1994;370(6488):341–347. - PubMed
-
- ten Dijke P, Miyazono K, Heldin C-H. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biol Sci. 2000;25(2):64–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

