Minimal cross-intolerance with nilotinib in patients with chronic myeloid leukemia in chronic or accelerated phase who are intolerant to imatinib
- PMID: 21467546
- PMCID: PMC4186645
- DOI: 10.1182/blood-2010-11-318949
Minimal cross-intolerance with nilotinib in patients with chronic myeloid leukemia in chronic or accelerated phase who are intolerant to imatinib
Abstract
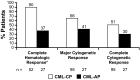
Nilotinib has significant efficacy in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) and in patients with CML-CP or CML in accelerated phase (CML-AP) after imatinib failure. We investigated the occurrence of cross-intolerance to nilotinib in imatinib-intolerant patients with CML. Only 1/75 (1%) patients with nonhematologic imatinib intolerance experienced a similar grade 3/4 adverse event (AE), and 3/75 (4%) experienced a similar persistent grade 2 nonhematologic AE on nilotinib. Only 7/40 (18%) patients with hematologic imatinib intolerance discontinued nilotinib, all because of grade 3/4 thrombocytopenia. Ninety percent of imatinib-intolerant patients with CML-CP who did not have complete hematologic response (CHR) at baseline (n = 52) achieved CHR on nilotinib. Nilotinib induced a major cytogenetic response in 66% and 41% of patients with imatinib-intolerant CML-CP and CML-AP (complete cytogenetic response in 51% and 30%), respectively. Minimal cross-intolerance was confirmed in patients with imatinib-intolerant CML. The favorable tolerability of nilotinib in patients with imatinib intolerance leads to alleviation of AE-related symptoms and significant and durable responses. In addition to its established clinical benefit in patients with newly diagnosed CML and those resistant to imatinib, nilotinib is effective and well-tolerated for long-term use in patients with imatinib intolerance. This study is registered at http://www.clinicaltrials.gov as NCT00471497.
Figures
References
-
- Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1084–1086. - PubMed
-
- Kavalerchik E, Goff D, Jamieson CH. Chronic myeloid leukemia stem cells. J Clin Oncol. 2008;26(17):2911–2915. - PubMed
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340(17):1330–1340. - PubMed
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. - PubMed
-
- Manley PW, Drueckes P, Fendrich G, et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804(3):445–453. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous