MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula
- PMID: 21467581
- PMCID: PMC3101557
- DOI: 10.1105/tpc.110.080804
MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula
Abstract
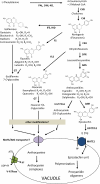
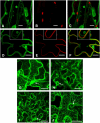
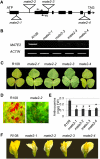
The majority of flavonoids, such as anthocyanins, proanthocyanidins, and isoflavones, are stored in the central vacuole, but the molecular basis of flavonoid transport is still poorly understood. Here, we report the functional characterization of a multidrug and toxin extrusion transporter (MATE2), from Medicago truncatula. MATE 2 is expressed primarily in leaves and flowers. Despite its high similarity to the epicatechin 3'-O-glucoside transporter MATE1, MATE2 cannot efficiently transport proanthocyanidin precursors. In contrast, MATE2 shows higher transport capacity for anthocyanins and lower efficiency for other flavonoid glycosides. Three malonyltransferases that are coexpressed with MATE2 were identified. The malonylated flavonoid glucosides generated by these malonyltransferases are more efficiently taken up into MATE2-containing membrane vesicles than are the parent glycosides. Malonylation increases both the affinity and transport efficiency of flavonoid glucosides for uptake by MATE2. Genetic loss of MATE2 function leads to the disappearance of leaf anthocyanin pigmentation and pale flower color as a result of drastic decreases in the levels of various flavonoids. However, some flavonoid glycoside malonates accumulate to higher levels in MATE2 knockouts than in wild-type controls. Deletion of MATE2 increases seed proanthocyanidin biosynthesis, presumably via redirection of metabolic flux from anthocyanin storage.
Figures





Similar articles
-
MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis.Plant Cell. 2009 Aug;21(8):2323-40. doi: 10.1105/tpc.109.067819. Epub 2009 Aug 14. Plant Cell. 2009. PMID: 19684242 Free PMC article.
-
Insight into the role of anthocyanin biosynthesis-related genes in Medicago truncatula mutants impaired in pigmentation in leaves.Plant Physiol Biochem. 2013 Sep;70:123-32. doi: 10.1016/j.plaphy.2013.05.030. Epub 2013 May 31. Plant Physiol Biochem. 2013. PMID: 23774374
-
Nucleocytoplasmic-localized acyltransferases catalyze the malonylation of 7-O-glycosidic (iso)flavones in Medicago truncatula.Plant J. 2008 Aug;55(3):382-96. doi: 10.1111/j.0960-7412.2008.03509.x. Plant J. 2008. PMID: 18419782
-
Functional Genomics in the Study of Metabolic Pathways in Medicago truncatula: An Overview.Methods Mol Biol. 2018;1822:315-337. doi: 10.1007/978-1-4939-8633-0_20. Methods Mol Biol. 2018. PMID: 30043312 Review.
-
Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration.Plant Cell Physiol. 2015 Jan;56(1):28-40. doi: 10.1093/pcp/pcu097. Epub 2014 Jul 10. Plant Cell Physiol. 2015. PMID: 25015943 Review.
Cited by
-
A glutathione S-transferase GhTT19 determines flower petal pigmentation via regulating anthocyanin accumulation in cotton.Plant Biotechnol J. 2023 Feb;21(2):433-448. doi: 10.1111/pbi.13965. Epub 2022 Dec 12. Plant Biotechnol J. 2023. PMID: 36385569 Free PMC article.
-
Computational and Transcriptomic Analysis Unraveled OsMATE34 as a Putative Anthocyanin Transporter in Black Rice (Oryza sativa L.) Caryopsis.Genes (Basel). 2021 Apr 16;12(4):583. doi: 10.3390/genes12040583. Genes (Basel). 2021. PMID: 33923742 Free PMC article.
-
Fruit ripening: dynamics and integrated analysis of carotenoids and anthocyanins.BMC Plant Biol. 2022 Jan 11;22(1):27. doi: 10.1186/s12870-021-03411-w. BMC Plant Biol. 2022. PMID: 35016620 Free PMC article. Review.
-
MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides.Plant Cell. 2016 Feb;28(2):583-96. doi: 10.1105/tpc.15.00706. Epub 2016 Jan 7. Plant Cell. 2016. PMID: 26744218 Free PMC article.
-
LhGST is an anthocyanin-related glutathione S-transferase gene in Asiatic hybrid lilies (Lilium spp.).Plant Cell Rep. 2021 Jan;40(1):85-95. doi: 10.1007/s00299-020-02615-y. Epub 2020 Nov 19. Plant Cell Rep. 2021. PMID: 33210154
References
-
- Ariga T., Asao Y., Sugimoto H., Yokotuska T. (1981). Occurrence of astringent oligomeric proanthocyanidins in legume seeds. Agric. Biol. Chem. 45: 2705–2708
-
- Benedito V.A., et al. (2008). A gene expression atlas of the model legume Medicago truncatula. Plant J. 55: 504–513 - PubMed
-
- Boller T., Wiemken A. (1986). Dynamics of vacuolar compartmentation. Annu. Rev. Plant Physiol. 37: 137–164
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

