Frontier of epilepsy research - mTOR signaling pathway
- PMID: 21467839
- PMCID: PMC3104248
- DOI: 10.3858/emm.2011.43.5.032
Frontier of epilepsy research - mTOR signaling pathway
Abstract
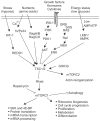
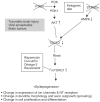
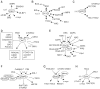
Studies of epilepsy have mainly focused on the membrane proteins that control neuronal excitability. Recently, attention has been shifting to intracellular proteins and their interactions, signaling cascades and feedback regulation as they relate to epilepsy. The mTOR (mammalian target of rapamycin) signal transduction pathway, especially, has been suggested to play an important role in this regard. These pathways are involved in major physiological processes as well as in numerous pathological conditions. Here, involvement of the mTOR pathway in epilepsy will be reviewed by presenting; an overview of the pathway, a brief description of key signaling molecules, a summary of independent reports and possible implications of abnormalities of those molecules in epilepsy, a discussion of the lack of experimental data, and questions raised for the understanding its epileptogenic mechanism.
Figures
References
-
- Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. - PubMed
-
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudière E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. - PubMed
-
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. - PubMed
-
- Albert L, Katsy M, Murali R, Jhanwar-Uniyal M. Inhibition of mTOR activates the MAPK pathway in glioblastoma multiforme. Cancer Genomics Proteomics. 2009;6:255–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
