GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils
- PMID: 21467997
- PMCID: PMC3132458
- DOI: 10.1038/cr.2011.60
GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils
Erratum in
- Cell Res. 2011 Nov;21(11):1641
Abstract
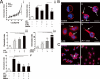
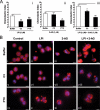
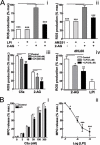
The directional migration of neutrophils towards inflammatory mediators, such as chemokines and cannabinoids, occurs via the activation of seven transmembrane G protein coupled receptors (7TM/GPCRs) and is a highly organized process. A crucial role for controlling neutrophil migration has been ascribed to the cannabinoid CB(2) receptor (CB(2)R), but additional modulatory sites distinct from CB(2)R have recently been suggested to impact CB(2)R-mediated effector functions in neutrophils. Here, we provide evidence that the recently de-orphanized 7TM/GPCR GPR55 potently modulates CB(2)R-mediated responses. We show that GPR55 is expressed in human blood neutrophils and its activation augments the migratory response towards the CB(2)R agonist 2-arachidonoylglycerol (2-AG), while inhibiting neutrophil degranulation and reactive oxygen species (ROS) production. Using HEK293 and HL60 cell lines, along with primary neutrophils, we show that GPR55 and CB(2)R interfere with each other's signaling pathways at the level of small GTPases, such as Rac2 and Cdc42. This ultimately leads to cellular polarization and efficient migration as well as abrogation of degranulation and ROS formation in neutrophils. Therefore, GPR55 limits the tissue-injuring inflammatory responses mediated by CB(2)R, while it synergizes with CB(2)R in recruiting neutrophils to sites of inflammation.
Figures
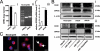


Similar articles
-
Involvement of the 90-kDa heat shock protein (Hsp-90) in CB2 cannabinoid receptor-mediated cell migration: a new role of Hsp-90 in migration signaling of a G protein-coupled receptor.Mol Pharmacol. 2007 Nov;72(5):1289-300. doi: 10.1124/mol.107.036566. Epub 2007 Aug 14. Mol Pharmacol. 2007. PMID: 17698952
-
The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55.J Neuroimmune Pharmacol. 2012 Dec;7(4):856-65. doi: 10.1007/s11481-012-9351-6. Epub 2012 Mar 28. J Neuroimmune Pharmacol. 2012. PMID: 22454039 Free PMC article.
-
Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils.J Biol Chem. 2006 May 5;281(18):12908-18. doi: 10.1074/jbc.M510871200. Epub 2006 Mar 2. J Biol Chem. 2006. PMID: 16513651
-
Is GPR55 an anandamide receptor?Vitam Horm. 2009;81:111-37. doi: 10.1016/S0083-6729(09)81005-4. Vitam Horm. 2009. PMID: 19647110 Review.
-
International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂.Pharmacol Rev. 2010 Dec;62(4):588-631. doi: 10.1124/pr.110.003004. Pharmacol Rev. 2010. PMID: 21079038 Free PMC article. Review.
Cited by
-
Cannabis-Based Phytocannabinoids: Overview, Mechanism of Action, Therapeutic Application, Production, and Affecting Environmental Factors.Int J Mol Sci. 2024 Oct 19;25(20):11258. doi: 10.3390/ijms252011258. Int J Mol Sci. 2024. PMID: 39457041 Free PMC article. Review.
-
Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer.Oncotarget. 2016 Jul 26;7(30):47565-47575. doi: 10.18632/oncotarget.10206. Oncotarget. 2016. PMID: 27340777 Free PMC article.
-
New blood brothers: the GPR55 and CB2 partnership.Cell Res. 2011 Oct;21(10):1391-2. doi: 10.1038/cr.2011.77. Epub 2011 May 3. Cell Res. 2011. PMID: 21537344 Free PMC article. Review. No abstract available.
-
Identification of co-expressed gene signatures in mouse B1, marginal zone and B2 B-cell populations.Immunology. 2014 Jan;141(1):79-95. doi: 10.1111/imm.12171. Immunology. 2014. PMID: 24032749 Free PMC article.
-
The Interplay between the Immune and the Endocannabinoid Systems in Cancer.Cells. 2021 May 21;10(6):1282. doi: 10.3390/cells10061282. Cells. 2021. PMID: 34064197 Free PMC article. Review.
References
-
- Chakravarti A, Allaeys I, Poubelle PE. Neutrophils and immunity: is it innate or acquired. Med Sci (Paris) 2007;23:862–867. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Witko-Sarsat V, Rieu P, scamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. - PubMed
-
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

