Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair
- PMID: 21468022
- PMCID: PMC3104517
- DOI: 10.1038/ncomms1270
Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair
Abstract
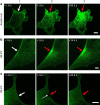
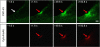
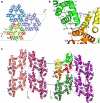
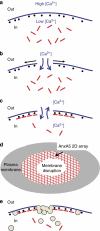
Eukaryotic cells possess a universal repair machinery that ensures rapid resealing of plasma membrane disruptions. Before resealing, the torn membrane is submitted to considerable tension, which functions to expand the disruption. Here we show that annexin-A5 (AnxA5), a protein that self-assembles into two-dimensional (2D) arrays on membranes upon Ca(2+) activation, promotes membrane repair. Compared with wild-type mouse perivascular cells, AnxA5-null cells exhibit a severe membrane repair defect. Membrane repair in AnxA5-null cells is rescued by addition of AnxA5, which binds exclusively to disrupted membrane areas. In contrast, an AnxA5 mutant that lacks the ability of forming 2D arrays is unable to promote membrane repair. We propose that AnxA5 participates in a previously unrecognized step of the membrane repair process: triggered by the local influx of Ca(2+), AnxA5 proteins bind to torn membrane edges and form a 2D array, which prevents wound expansion and promotes membrane resealing.
© 2011 Macmillan Publishers Limited. All rights reserved.
Figures
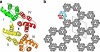


References
-
- McNeil P. L. & Steinhardt R. A. Plasma membrane disruption: repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 19, 697–731 (2003). - PubMed
-
- Glover L. & Brown R. H. Dysferlin in membrane trafficking and patch repair. Traffic 8, 785–794 (2007). - PubMed
-
- McNeil P. L. & Kirchhausen T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6, 499–505 (2005). - PubMed
-
- Steinhardt R. A., Bi G. & Alderton J. M. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263, 390–393 (1994). - PubMed
-
- Sheetz M. P. & Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 6, 85–89 (1996). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

