Physiological origin for the BOLD poststimulus undershoot in human brain: vascular compliance versus oxygen metabolism
- PMID: 21468090
- PMCID: PMC3137471
- DOI: 10.1038/jcbfm.2011.35
Physiological origin for the BOLD poststimulus undershoot in human brain: vascular compliance versus oxygen metabolism
Abstract
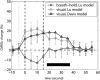
The poststimulus blood oxygenation level-dependent (BOLD) undershoot has been attributed to two main plausible origins: delayed vascular compliance based on delayed cerebral blood volume (CBV) recovery and a sustained increased oxygen metabolism after stimulus cessation. To investigate these contributions, multimodal functional magnetic resonance imaging was employed to monitor responses of BOLD, cerebral blood flow (CBF), total CBV, and arterial CBV (CBV(a)) in human visual cortex after brief breath hold and visual stimulation. In visual experiments, after stimulus cessation, CBV(a) was restored to baseline in 7.9±3.4 seconds, and CBF and CBV in 14.8±5.0 seconds and 16.1±5.8 seconds, respectively, all significantly faster than BOLD signal recovery after undershoot (28.1±5.5 seconds). During the BOLD undershoot, postarterial CBV (CBV(pa), capillaries and venules) was slightly elevated (2.4±1.8%), and cerebral metabolic rate of oxygen (CMRO(2)) was above baseline (10.6±7.4%). Following breath hold, however, CBF, CBV, CBV(a) and BOLD signals all returned to baseline in ∼20 seconds. No significant BOLD undershoot, and residual CBV(pa) dilation were observed, and CMRO(2) did not substantially differ from baseline. These data suggest that both delayed CBV(pa) recovery and enduring increased oxidative metabolism impact the BOLD undershoot. Using a biophysical model, their relative contributions were estimated to be 19.7±15.9% and 78.7±18.6%, respectively.
Figures



References
-
- Blockley NP, Francis ST, Gowland PA. Perturbation of the BOLD response by a contrast agent and interpretation through a modified balloon model. NeuroImage. 2009;48:84. - PubMed
-
- Buxton RB. Proc 17th Annual Meeting ISMRM. Hawaii, USA; 2009. Modeling the effect of changes in arterial blood volume on the BOLD signal; p. 1620.
-
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855. - PubMed
-
- Chen JJ, Pike GB. Origins of the BOLD post-stimulus undershoot. NeuroImage. 2009;46:559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

