Trypanosoma cruzi in the chicken model: Chagas-like heart disease in the absence of parasitism
- PMID: 21468314
- PMCID: PMC3066158
- DOI: 10.1371/journal.pntd.0001000
Trypanosoma cruzi in the chicken model: Chagas-like heart disease in the absence of parasitism
Abstract
Background: The administration of anti-trypanosome nitroderivatives curtails Trypanosoma cruzi infection in Chagas disease patients, but does not prevent destructive lesions in the heart. This observation suggests that an effective treatment for the disease requires understanding its pathogenesis.
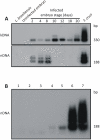
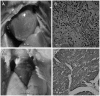
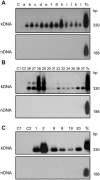
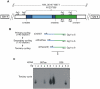
Methodology/principal findings: To understand the origin of clinical manifestations of the heart disease we used a chicken model system in which infection can be initiated in the egg, but parasite persistence is precluded. T. cruzi inoculation into the air chamber of embryonated chicken eggs generated chicks that retained only the parasite mitochondrial kinetoplast DNA minicircle in their genome after eight days of gestation. Crossbreeding showed that minicircles were transferred vertically via the germ line to chicken progeny. Minicircle integration in coding regions was shown by targeted-primer thermal asymmetric interlaced PCR, and detected by direct genomic analysis. The kDNA-mutated chickens died with arrhythmias, shortness of breath, cyanosis and heart failure. These chickens with cardiomyopathy had rupture of the dystrophin and other genes that regulate cell growth and differentiation. Tissue pathology revealed inflammatory dilated cardiomegaly whereby immune system mononuclear cells lyse parasite-free target heart fibers. The heart cell destruction implicated a thymus-dependent, autoimmune; self-tissue rejection carried out by CD45(+), CD8γδ(+), and CD8α lymphocytes.
Conclusions/significance: These results suggest that genetic alterations resulting from kDNA integration in the host genome lead to autoimmune-mediated destruction of heart tissue in the absence of T. cruzi parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Pathogenesis of chagas' disease: parasite persistence and autoimmunity.Clin Microbiol Rev. 2011 Jul;24(3):592-630. doi: 10.1128/CMR.00063-10. Clin Microbiol Rev. 2011. PMID: 21734249 Free PMC article. Review.
-
Parasite induced genetically driven autoimmune Chagas heart disease in the chicken model.J Vis Exp. 2012 Jul 29;(65):3716. doi: 10.3791/3716. J Vis Exp. 2012. PMID: 22951533 Free PMC article.
-
Inhibition of autoimmune Chagas-like heart disease by bone marrow transplantation.PLoS Negl Trop Dis. 2014 Dec 18;8(12):e3384. doi: 10.1371/journal.pntd.0003384. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25521296 Free PMC article.
-
Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease.Mol Biochem Parasitol. 1991 Oct;48(2):211-21. doi: 10.1016/0166-6851(91)90116-n. Mol Biochem Parasitol. 1991. PMID: 1662334
-
Autoimmunity.Adv Parasitol. 2011;76:129-52. doi: 10.1016/B978-0-12-385895-5.00006-2. Adv Parasitol. 2011. PMID: 21884890 Review.
Cited by
-
Pathogenesis of chagas' disease: parasite persistence and autoimmunity.Clin Microbiol Rev. 2011 Jul;24(3):592-630. doi: 10.1128/CMR.00063-10. Clin Microbiol Rev. 2011. PMID: 21734249 Free PMC article. Review.
-
Oral administration of GW788388, an inhibitor of transforming growth factor beta signaling, prevents heart fibrosis in Chagas disease.PLoS Negl Trop Dis. 2012;6(6):e1696. doi: 10.1371/journal.pntd.0001696. Epub 2012 Jun 12. PLoS Negl Trop Dis. 2012. Retraction in: PLoS Negl Trop Dis. 2024 Jul 23;18(7):e0012360. doi: 10.1371/journal.pntd.0012360. PMID: 22720109 Free PMC article. Retracted.
-
Translational challenges of animal models in Chagas disease drug development: a review.Drug Des Devel Ther. 2015 Aug 19;9:4807-23. doi: 10.2147/DDDT.S90208. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26316715 Free PMC article. Review.
-
Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells.Parasitol Res. 2014 Jan;113(1):285-304. doi: 10.1007/s00436-013-3655-1. Epub 2013 Nov 17. Parasitol Res. 2014. PMID: 24241124
-
Correlation of Parasite Burden, kDNA Integration, Autoreactive Antibodies, and Cytokine Pattern in the Pathophysiology of Chagas Disease.Front Microbiol. 2019 Aug 21;10:1856. doi: 10.3389/fmicb.2019.01856. eCollection 2019. Front Microbiol. 2019. PMID: 31496999 Free PMC article.
References
-
- Bittencourt AL. Congenital Chagas disease. Am J Dis Child. 1976;130(1):97–103. - PubMed
-
- Bittencourt AL. Possible risk factors for vertical transmission of Chagas' disease. Rev Inst Med Trop Sao Paulo. 1992;34(5):403–408. - PubMed
-
- Bittencourt AL, Barbosa HS. Importance of the study of the macerated fetus for the diagnosis of congenital Chagas' disease. Rev Inst Med Trop Sao Paulo. 1972;14(4):260–263. - PubMed
-
- Bittencourt AL, Barbosa HS. Incidence of congenital transmission of Chagas' disease in abortion. Rev Inst Med Trop Sao Paulo. 1972;14(4):257–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

