TLR2 signaling improves immunoregulation to prevent type 1 diabetes
- PMID: 21469083
- PMCID: PMC3100206
- DOI: 10.1002/eji.200939841
TLR2 signaling improves immunoregulation to prevent type 1 diabetes
Abstract
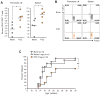
Signaling through TLR2 promotes inflammation and modulates CD4(+) CD25(+) Tregs. We assessed mechanistically how this molecule would alter immunoregulation in type 1 diabetes (T1D). We also asked whether TLR2 may be involved in our recent discovery that viral infection can protect from autoimmune diabetes by expanding and invigorating Tregs. Treatment of prediabetic mice with a synthetic TLR2 agonist diminished T1D and increased the number and function of CD4(+) CD25(+) Tregs, also conferring DCs with tolerogenic properties. TLR2 ligation also promoted the expansion of Tregs upon culture with DCs and ameliorated their capacity to prevent the disease. Protection from T1D by lymphocytic choriomeningitis virus (LCMV) infection depended on TLR2. LCMV increased the frequency of CD4(+) CD25(+) Tregs and their production of TGF-β more significantly in WT than TLR2-deficient mice. Furthermore, LCMV infection in vivo or LCMV-infected DCs in vitro rendered, via TLR2, CD4(+) CD25(+) Tregs capable of diminishing T1D. We identify novel mechanisms by which TLR2 promotes immunoregulation and controls autoimmune diabetes in naïve or infected hosts. This work should help understand T1D etiology and develop novel immune-based therapeutic interventions.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. - PubMed
-
- Foulis AK, Jackson R, Farquharson MA. The pancreas in idiopathic Addison’s disease--a search for a prediabetic pancreas. Histopathology. 1988;12:481–490. - PubMed
-
- Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110:134–144. - PubMed
-
- Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

