Diffusion tensor imaging and beyond
- PMID: 21469191
- PMCID: PMC3366862
- DOI: 10.1002/mrm.22924
Diffusion tensor imaging and beyond
Figures
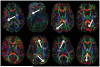
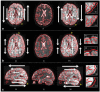
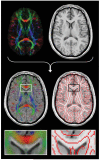
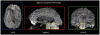
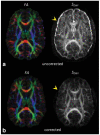
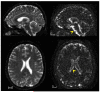
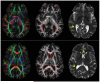

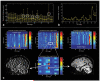
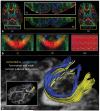


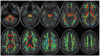


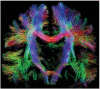
References
-
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. - PubMed
-
- Le Bihan D, Turner R, MacFall J. Effects of intravoxel incoherent motions (IVIM) in steady-state free precession (SSFP) imaging: application to molecular diffusion imaging. Magn Reson Med. 1989;10:324–337. - PubMed
-
- Merboldt KD, Bruhn H, Frahm J, Gyngell ML, Hanicke W, Deimling M. MRI of “diffusion” in the human brain: new results using a modified CE-FAST sequence. Magn Reson Med. 1989;9:423–429. - PubMed
-
- Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: demonstration with MR technique in vivo. Radiology. 1990;177:401–405. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

