C(21)-C(40) of tetrafibricin via metal catalysis: beyond stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles
- PMID: 21469726
- PMCID: PMC3084888
- DOI: 10.1021/ol200735r
C(21)-C(40) of tetrafibricin via metal catalysis: beyond stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles
Abstract
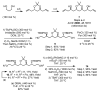
The C(21)-C(40) fragment of fibrinogen receptor inhibitor tetrafibricin was prepared in 12 steps from propane diol (longest linear sequence). In this approach, 6 C-C bonds are formed via asymmetric iridium catalyzed transfer hydrogenative carbonyl allylation and 2 C═C bonds are formed via Grubbs olefin cross-metathesis.
Figures
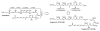



Similar articles
-
Asymmetric alcohol C-H allylation and syn-crotylation: C9-C20 of tetrafibricin.Org Lett. 2014 Feb 7;16(3):820-3. doi: 10.1021/ol403566w. Epub 2014 Jan 14. Org Lett. 2014. PMID: 24422777 Free PMC article.
-
Total synthesis of (+)-roxaticin via C-C bond forming transfer hydrogenation: a departure from stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles in polyketide construction.J Am Chem Soc. 2010 Nov 10;132(44):15559-61. doi: 10.1021/ja1082798. J Am Chem Soc. 2010. PMID: 20961111 Free PMC article.
-
Total Synthesis of the Acetyl CoA Carboxylase Inhibitor Soraphen A: Asymmetric Tsuji Reduction Enables Successive Olefin Metathesis.J Am Chem Soc. 2022 Jan 19;144(2):1016-1022. doi: 10.1021/jacs.1c12063. Epub 2022 Jan 10. J Am Chem Soc. 2022. PMID: 35005976 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency.Chem Commun (Camb). 2009 Dec 21;(47):7278-87. doi: 10.1039/b917243m. Epub 2009 Oct 16. Chem Commun (Camb). 2009. PMID: 20024203 Free PMC article. Review.
-
Asymmetric Iridium-Catalyzed C-C Coupling of Chiral Diols via Site-Selective Redox-Triggered Carbonyl Addition.Top Curr Chem. 2016;372:85-101. doi: 10.1007/128_2015_651. Top Curr Chem. 2016. PMID: 26187028 Free PMC article. Review.
Cited by
-
Enantio- and diastereoselective synthesis of N-acetyl dihydrotetrafibricin methyl ester.J Am Chem Soc. 2013 Apr 10;135(14):5340-3. doi: 10.1021/ja401918r. Epub 2013 Mar 26. J Am Chem Soc. 2013. PMID: 23530855 Free PMC article.
-
Asymmetric alcohol C-H allylation and syn-crotylation: C9-C20 of tetrafibricin.Org Lett. 2014 Feb 7;16(3):820-3. doi: 10.1021/ol403566w. Epub 2014 Jan 14. Org Lett. 2014. PMID: 24422777 Free PMC article.
-
Stereocontrolled Total Synthesis of Bastimolide B Using Iterative Homologation of Boronic Esters.J Am Chem Soc. 2022 May 11;144(18):7995-8001. doi: 10.1021/jacs.2c03192. Epub 2022 May 2. J Am Chem Soc. 2022. PMID: 35499478 Free PMC article.
-
Development of a Double Allylboration Reagent Targeting 1,5-syn-(E)-Diols: Application to the Synthesis of the C(23)-C(40) Fragment of Tetrafibricin.Tetrahedron. 2011 Sep 2;67(35):6497-6512. doi: 10.1016/j.tet.2011.06.008. Tetrahedron. 2011. PMID: 21857752 Free PMC article.
References
-
-
For selected reviews on C-C bond forming hydrogenation and transfer hydrogenation, see: Patman RL, Bower JF, Kim IS, Krische MJ. Aldrichim. Acta. 2008;41:95. Bower JF, Kim IS, Patman RL, Krische MJ. Angew. Chem. Int. Ed. 2009;48:34. Han SB, Kim IS, Krische MJ. Chem. Commun. 2009:7278. Bower JF, Krische MJ. Top. Organomet. Chem. 2011;43:107.
-
-
-
In related “hydrogen auto-transfer” or “borrowing hydrogen” processes, alcohol dehydrogenation and nucleophile generation occur independently. Hence, conventional pre-activated nucleophiles are required. Such processes deliver products of formal alcohol substitution rather than carbonyl addition. For selected reviews, see: Guillena G, Ramón DJ, Yus M. Angew. Chem. Int. Ed. 2007;46:2358. Hamid MHSA, Slatford PA, Williams JMJ. Adv. Synth. Catal. 2007;349:1555. Nixon TD, Whittlesey MK, Williams JMJ. Dalton Trans. 2009:753. Dobereiner GE, Crabtree RH. Chem. Rev. 2010;110:681. Guillena G, Ramón DJ, Yus M. Chem. Rev. 2010;110:1611. Li C-J. Acc. Chem. Res. 2009;42:335.
-
-
-
For applications in total synthesis, see: Lu Y, Krische MJ. Org. Lett. 2009;11:3108. Harsh P, O'Doherty GA. Tetrahedron. 2009;65:5051. Sawant P, Maier ME. Tetrahedron. 2010;66:9738. Han SB, Hassan A, Kim I-S, Krische MJ. J. Am. Chem. Soc. 2010;132:15559.
-
-
- Kim IS, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2008;130:6340. - PMC - PubMed
- Kim IS, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2008;130:14891. - PMC - PubMed
- Lu Y, Kim IS, Hassan A, Del Valle DJ, Krische MJ. Angew. Chem., Int. Ed. 2009;48:5018. - PMC - PubMed
- Hassan A, Lu Y, Krische MJ. Org. Lett. 2009;11:3112. - PMC - PubMed
-
-
For isolation and stereochemical assignment, see: Kamiyama T, Umino T, Fujisaki N, Satoh T, Yamashita Y, Ohshima S, Watanabe J, Yokose K. J. Antibiot. 1993;46:1039. Kamiyama T, Itezono Y, Umino T, Satoh T, Nakayama N, Yokose K. J. Antibiot. 1993;46:1047. Kobayashi Y, Czechtizky W, Kishi Y. Org. Lett. 2003;5:93.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

