Phytobacterial type III effectors HopX1, HopAB1 and HopF2 enhance sense-post-transcriptional gene silencing independently of plant R gene-effector recognition
- PMID: 21469938
- PMCID: PMC3788636
- DOI: 10.1094/MPMI-01-11-0010
Phytobacterial type III effectors HopX1, HopAB1 and HopF2 enhance sense-post-transcriptional gene silencing independently of plant R gene-effector recognition
Erratum in
- Mol Plant Microbe Interact. 2012 Mar;25(3):440
Abstract
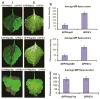
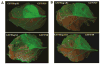
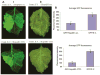
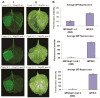
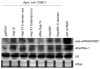
Plant- and animal-pathogenic bacteria deploy a variable arsenal of type III effector proteins (T3EP) to manipulate host defense. Specific biochemical functions and molecular or subcellular targets have been demonstrated or proposed for a growing number of T3EP but remain unknown for the majority of them. Here, we show that transient expression of genes coding certain bacterial T3EP (HopAB1, HopX1, and HopF2), which did not elicit hypersensitive response (HR) in transgenic green fluorescent protein (GFP) Nicotiana benthamiana 16C line, enhanced the sense post-transcriptional gene silencing (S-PTGS) triggered by agrodelivery of a GFP-expressing cassette and the silencing enhancement could be blocked by two well-known viral silencing suppressors. Further analysis using genetic truncations and site-directed mutations showed that the receptor recognition domains of HopAB1 and HopX1 are not involved in enhancing silencing. Our studies provide new evidence that phytobacterial pathogen T3EP manipulate the plant small interfering RNA pathways by enhancing silencing efficiency in the absence of effector-triggered immunity signaling and suggest that phytopathogenic bacterial effectors affect host RNA silencing in yet other ways than previously described.
Figures
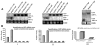

References
-
- Abramovitch RB, Martin GB. AvrPtoB: A bacterial type III effector that both elicits and suppresses programmed cell death associated with plant immunity. FEMS (Fed Eur Microbiol Soc) Microbiol Lett. 2005;245:1–8. - PubMed
-
- Alfano JR, Collmer A. Type III secretion system effector proteins: Double agents in bacterial disease and plant defense. Annu Rev Phytopathol. 2004;42:385–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

