Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors
- PMID: 21470208
- PMCID: PMC3423240
- DOI: 10.1111/j.1476-5381.2011.01410.x
Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors
Abstract
Background and purpose: Activation of cannabinoid CB(2) receptors protects against various forms of ischaemia-reperfusion (I/R) injury. Δ(8) -Tetrahydrocannabivarin (Δ(8) -THCV) is a synthetic analogue of the plant cannabinoid Δ(9) -tetrahydrocannabivarin, which exhibits anti-inflammatory effects in rodents involving activation of CB(2) receptors. Here, we assessed effects of Δ(8) -THCV and its metabolite 11-OH-Δ(8) -THCV on CB(2) receptors and against hepatic I/R injury.
Experimental approach: Effects in vitro were measured with human CB(2) receptors expressed in CHO cells. Hepatic I/R injury was assessed in mice with 1h ischaemia and 2, 6 or 24h reperfusion in vivo.
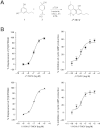
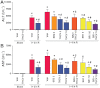
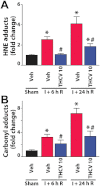
Key results: Displacement of [(3) H]CP55940 by Δ(8) -THCV or 11-OH-Δ(8) -THCV from specific binding sites in CHO cell membranes transfected with human CB(2) receptors (hCB(2) ) yielded K(i) values of 68.4 and 59.95 nM respectively. Δ(8) -THCV or 11-OH-Δ(8) -THCV inhibited forskolin-stimulated cAMP production by hCB(2) CHO cells (EC(50) = 12.95 and 14.3 nM respectively). Δ(8) -THCV, given before induction of I/R, attenuated hepatic injury (measured by serum alanine aminotransferase and aspartate aminotransferase levels), decreased tissue protein carbonyl adducts, 4-hydroxy-2-nonenal, the chemokines CCL3 and CXCL2,TNF-α, intercellular adhesion molecule 1 (CD54) mRNA levels, tissue neutrophil infiltration, caspase 3/7 activity and DNA fragmentation. Protective effects of Δ(8) -THCV against liver damage were still present when the compound was given at the beginning of reperfusion. Pretreatment with a CB(2) receptor antagonist attenuated the protective effects of Δ(8) -THCV, while a CB(1) antagonist tended to enhance it.
Conclusions and implications: Δ(8) -THCV activated CB(2) receptors in vitro, and decreased tissue injury and inflammation in vivo, associated with I/R partly via CB(2) receptor activation.
Linked articles: This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7.
Published 2011. This article is a U.S. Government work and is in the public domain in the USA.
Figures





Similar articles
-
A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury.Br J Pharmacol. 2012 Apr;165(8):2462-78. doi: 10.1111/j.1476-5381.2011.01381.x. Br J Pharmacol. 2012. PMID: 21449982 Free PMC article.
-
The plant cannabinoid Delta9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice.Br J Pharmacol. 2010 Jun;160(3):677-87. doi: 10.1111/j.1476-5381.2010.00756.x. Br J Pharmacol. 2010. PMID: 20590571 Free PMC article.
-
AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor.Br J Pharmacol. 2012 Apr;165(8):2561-74. doi: 10.1111/j.1476-5381.2011.01503.x. Br J Pharmacol. 2012. PMID: 21615724 Free PMC article.
-
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.Br J Pharmacol. 2008 Jan;153(2):199-215. doi: 10.1038/sj.bjp.0707442. Epub 2007 Sep 10. Br J Pharmacol. 2008. PMID: 17828291 Free PMC article. Review.
-
Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning.Br J Pharmacol. 2008 Jan;153(2):252-62. doi: 10.1038/sj.bjp.0707582. Epub 2007 Nov 19. Br J Pharmacol. 2008. PMID: 18026124 Free PMC article. Review.
Cited by
-
Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants.Pharmaceuticals (Basel). 2024 Nov 27;17(12):1603. doi: 10.3390/ph17121603. Pharmaceuticals (Basel). 2024. PMID: 39770444 Free PMC article.
-
Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions.J Neurosci. 2012 Mar 21;32(12):4004-16. doi: 10.1523/JNEUROSCI.4628-11.2012. J Neurosci. 2012. PMID: 22442067 Free PMC article.
-
Alcohol induced behavioral and immune perturbations are attenuated by activation of CB2 cannabinoid receptors.Adv Drug Alcohol Res. 2023 Dec 19;3:11602. doi: 10.3389/adar.2023.11602. eCollection 2023. Adv Drug Alcohol Res. 2023. PMID: 38389814 Free PMC article.
-
Anti-inflammatory mechanisms of cannabinoids: an immunometabolic perspective.Inflammopharmacology. 2019 Feb;27(1):39-46. doi: 10.1007/s10787-018-00560-7. Epub 2019 Jan 4. Inflammopharmacology. 2019. PMID: 30610735 Review.
-
Using In Silico Molecular Docking to Explain Differences in Receptor Binding Behavior of HHC and THCV Isomers: Revealing New Binding Modes.Pharmaceuticals (Basel). 2024 May 15;17(5):637. doi: 10.3390/ph17050637. Pharmaceuticals (Basel). 2024. PMID: 38794207 Free PMC article.
References
-
- Caraceni P, Pertosa AM, Giannone F, Domenicali M, Grattagliano I, Principe A, et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia–reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135–1143. - PubMed
-
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

