Corrosion of tungsten microelectrodes used in neural recording applications
- PMID: 21470563
- PMCID: PMC3107924
- DOI: 10.1016/j.jneumeth.2011.03.012
Corrosion of tungsten microelectrodes used in neural recording applications
Abstract
In neuroprosthetic applications, long-term electrode viability is necessary for robust recording of the activity of neural populations used for generating communication and control signals. The corrosion of tungsten microwire electrodes used for intracortical recording applications was analyzed in a controlled bench-top study and compared to the corrosion of tungsten microwires used in an in vivo study. Two electrolytes were investigated for the bench-top electrochemical analysis: 0.9% phosphate buffered saline (PBS) and 0.9% PBS containing 30 mM of hydrogen peroxide. The oxidation and reduction reactions responsible for corrosion were found by measurement of the open circuit potential and analysis of Pourbaix diagrams. Dissolution of tungsten to form the tungstic ion was found to be the corrosion mechanism. The corrosion rate was estimated from the polarization resistance, which was extrapolated from the electrochemical impedance spectroscopy data. The results show that tungsten microwires in an electrolyte of PBS have a corrosion rate of 300-700 μm/yr. The corrosion rate for tungsten microwires in an electrolyte containing PBS and 30 mM H₂O₂ is accelerated to 10,000-20,000 μm/yr. The corrosion rate was found to be controlled by the concentration of the reacting species in the cathodic reaction (e.g. O₂ and H₂O₂). The in vivo corrosion rate, averaged over the duration of implantation, was estimated to be 100 μm/yr. The reduced in vivo corrosion rate as compared to the bench-top rate is attributed to decreased rate of oxygen diffusion caused by the presence of a biological film and a reduced concentration of available oxygen in the brain.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






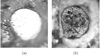











References
-
- Anik M. ph-dependent anodic reaction behavior of tungsten in acidic phosphate solutions. Electrochimica Acta. 2009;54(15):3943–3951.
-
- Anik M, Osseo-Asare K. Effect of ph on the anodic behavior of tungsten. Journal of The Electrochemical Society. 2002;149(6):B224–B233.
-
- Benson BB, Krause D., Jr. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnology and Oceanography. 1984;29:620–632.
-
- Bothra S, Sur H, Liang V. A new failure mechanism by corrosion of tungsten in a tungsten plug process. Microelectronics and Reliability. 1999;39(1):59–68.
-
- Brug G, den Eeden AV, Sluyters-Rehbach M, Sluyters J. The analysis of electrode impedances complicated by the presence of a constant phase element. Electroanal Chem. 1984;176:275–295.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

