Ex vivo functional responses to HLA-G differ between blood and decidual NK cells
- PMID: 21471023
- PMCID: PMC3160205
- DOI: 10.1093/molehr/gar022
Ex vivo functional responses to HLA-G differ between blood and decidual NK cells
Abstract
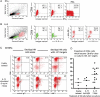
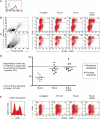
Restricted expression of human leucocyte antigen-G (HLA-G) to fetal extravillous trophoblast cells, which invade the decidua during implantation, suggests a role for HLA-G in placentation. In this study, we have investigated several aspects of HLA-G expression and function. Surface levels of HLA-G expression were measured in 70 normal pregnancies. We show the dimeric conformation that is unique to HLA-G forms after passage through the Golgi apparatus. Differences were found in the receptor repertoire of decidual natural killer (dNK) cells that express the leucocyte immunoglobulin-like receptor B1 (LILRB1), which binds dimeric HLA-G strongly. We then measured functional responses of dNK cells with LILRB1, when stimulated by HLA-G in both monomeric and dimeric conformations. Degranulation, interferon-γ and interleukin-8 production by dNK cells freshly isolated from the first trimester implantation site were either undetected or not affected by HLA-G. These findings should be considered when inferring the activity of tissue NK cells from results obtained with cell lines, peripheral NK or cultured dNK cells.
© The Author 2011. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved.
Figures



References
-
- Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. - PubMed
-
- Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. - PMC - PubMed
-
- Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. - PubMed
-
- Banham AH, Colonna M, Cella M, Micklem KJ, Pulford K, Willis AC, Mason DY. Identification of the CD85 antigen as ILT2, an inhibitory MHC class I receptor of the immunoglobulin superfamily. J Leukoc Biol. 1999;65:841–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

