Neisseria meningitidis adhesin NadA targets beta1 integrins: functional similarity to Yersinia invasin
- PMID: 21471204
- PMCID: PMC3121457
- DOI: 10.1074/jbc.M110.188326
Neisseria meningitidis adhesin NadA targets beta1 integrins: functional similarity to Yersinia invasin
Abstract
Meningococci are facultative-pathogenic bacteria endowed with a set of adhesins allowing colonization of the human upper respiratory tract, leading to fulminant meningitis and septicemia. The Neisseria adhesin NadA was identified in about 50% of N. meningitidis isolates and is closely related to the Yersinia adhesin YadA, the prototype of the oligomeric coiled-coil adhesin (Oca) family. NadA is known to be involved in cell adhesion, invasion, and induction of proinflammatory cytokines. Because of the enormous diversity of neisserial cell adhesins the analysis of the specific contribution of NadA in meningococcal host interactions is limited. Therefore, we used a non-invasive Y. enterocolitica mutant as carrier to study the role of NadA in host cell interaction. NadA was shown to be efficiently produced and localized in its oligomeric form on the bacterial surface of Y. enterocolitica. Additionally, NadA mediated a β1 integrin-dependent adherence with subsequent internalization of yersiniae by a β1 integrin-positive cell line. Using recombinant NadA(24-210) protein and human and murine β1 integrin-expressing cell lines we could demonstrate the role of the β1 integrin subunit as putative receptor for NadA. Subsequent inhibition assays revealed specific interaction of NadA(24-210) with the human β1 integrin subunit. Cumulatively, these results indicate that Y. enterocolitica is a suitable toolbox system for analysis of the adhesive properties of NadA, revealing strong evidence that β1 integrins are important receptors for NadA. Thus, this study demonstrated for the first time a direct interaction between the Oca-family member NadA and human β1 integrins.
Figures

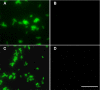

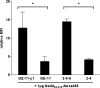
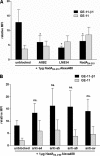



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

