Highly sulfated nonreducing end-derived heparan sulfate domains bind fibroblast growth factor-2 with high affinity and are enriched in biologically active fractions
- PMID: 21471211
- PMCID: PMC3103309
- DOI: 10.1074/jbc.M110.204693
Highly sulfated nonreducing end-derived heparan sulfate domains bind fibroblast growth factor-2 with high affinity and are enriched in biologically active fractions
Abstract
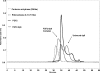
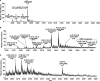
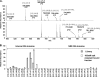
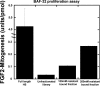
Human fibroblast growth factor-2 (FGF2) regulates cellular processes including proliferation, adhesion, motility, and angiogenesis. FGF2 exerts its biological function by binding and dimerizing its receptor (FGFR), which activates signal transduction cascades. Effective binding of FGF2 to its receptor requires the presence of heparan sulfate (HS), a linear polysaccharide with N-sulfated domains (NS) localized at the cell surface and extracellular matrix. HS acts as a platform facilitating the formation of a functional FGF-FGFR-HS ternary complex. Crystal structures of the signaling ternary complex revealed two conflicting architectures. In the asymmetrical model, two FGFs and two FGFRs bind a single HS chain. In contrast, the symmetrical model postulates that one FGF and one FGFR bind to the free end of the HS chain and dimerization require these ends to join, bringing the two half-complexes together. In this study, we screened a hexasaccharide HS library for compositions that are able to bind FGF2. The library was composed primarily of NS domains internal to the HS chain with minor presence of non-reducing end (NRE) NS. The binders were categorized into low versus high affinity binders. The low affinity fraction contained primarily hexasaccharides with low degree of sulfation that were internal to the HS chains. In contrast, the high affinity bound fraction was enriched in NRE oligosaccharides that were considerably more sulfated and had the ability to promote FGFR-mediated cell proliferation. The results suggest a role of the NRE of HS in FGF2 signaling and favor the formation of the symmetrical architecture on short NS domains.
Figures

Similar articles
-
Fibroblast growth factor-based signaling through synthetic heparan sulfate blocks copolymers studied using high cell density three-dimensional cell printing.J Biol Chem. 2014 Apr 4;289(14):9754-65. doi: 10.1074/jbc.M113.546937. Epub 2014 Feb 22. J Biol Chem. 2014. PMID: 24563485 Free PMC article.
-
Heparan Sulfate Domains Required for Fibroblast Growth Factor 1 and 2 Signaling through Fibroblast Growth Factor Receptor 1c.J Biol Chem. 2017 Feb 10;292(6):2495-2509. doi: 10.1074/jbc.M116.761585. Epub 2016 Dec 28. J Biol Chem. 2017. PMID: 28031461 Free PMC article.
-
Compositional analysis of heparin/heparan sulfate interacting with fibroblast growth factor.fibroblast growth factor receptor complexes.Biochemistry. 2009 Sep 8;48(35):8379-86. doi: 10.1021/bi9006379. Biochemistry. 2009. PMID: 19591432 Free PMC article.
-
Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer.Matrix Biol. 2010 Jul;29(6):442-52. doi: 10.1016/j.matbio.2010.04.003. Epub 2010 Apr 21. Matrix Biol. 2010. PMID: 20416374 Free PMC article. Review.
-
A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs.Curr Opin Struct Biol. 2005 Oct;15(5):506-16. doi: 10.1016/j.sbi.2005.09.002. Curr Opin Struct Biol. 2005. PMID: 16154740 Review.
Cited by
-
Structure and unusual binding mechanism of the hyaluronan receptor LYVE-1 mediating leucocyte entry to lymphatics.Nat Commun. 2025 Mar 20;16(1):2754. doi: 10.1038/s41467-025-57866-8. Nat Commun. 2025. PMID: 40113779 Free PMC article.
-
Oligosaccharide substrate preferences of human extracellular sulfatase Sulf2 using liquid chromatography-mass spectrometry based glycomics approaches.PLoS One. 2014 Aug 15;9(8):e105143. doi: 10.1371/journal.pone.0105143. eCollection 2014. PLoS One. 2014. PMID: 25127119 Free PMC article.
-
GlycReSoft: a software package for automated recognition of glycans from LC/MS data.PLoS One. 2012;7(9):e45474. doi: 10.1371/journal.pone.0045474. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049804 Free PMC article.
-
Gas-Phase Analysis of the Complex of Fibroblast GrowthFactor 1 with Heparan Sulfate: A Traveling Wave Ion Mobility Spectrometry (TWIMS) and Molecular Modeling Study.J Am Soc Mass Spectrom. 2017 Jan;28(1):96-109. doi: 10.1007/s13361-016-1496-8. Epub 2016 Sep 23. J Am Soc Mass Spectrom. 2017. PMID: 27663556 Free PMC article.
-
Tandem mass spectrometry of heparan sulfate negative ions: sulfate loss patterns and chemical modification methods for improvement of product ion profiles.J Am Soc Mass Spectrom. 2012 Sep;23(9):1498-511. doi: 10.1007/s13361-012-0429-4. Epub 2012 Jul 24. J Am Soc Mass Spectrom. 2012. PMID: 22825743 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

