Differential use of chondroitin sulfate to regulate hyaluronan binding by receptor CD44 in Inflammatory and Interleukin 4-activated Macrophages
- PMID: 21471214
- PMCID: PMC3103297
- DOI: 10.1074/jbc.M110.200790
Differential use of chondroitin sulfate to regulate hyaluronan binding by receptor CD44 in Inflammatory and Interleukin 4-activated Macrophages
Abstract
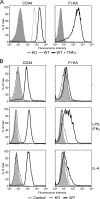
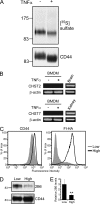
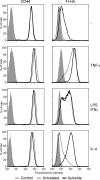
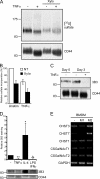
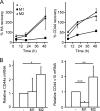
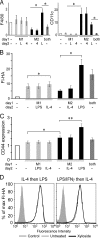
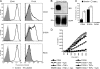
CD44 is a cell surface receptor for the extracellular matrix glycosaminoglycan hyaluronan and is involved in processes ranging from leukocyte recruitment to wound healing. In the immune system, the binding of hyaluronan to CD44 is tightly regulated, and exposure of human peripheral blood monocytes to inflammatory stimuli increases CD44 expression and induces hyaluronan binding. Here we sought to understand how mouse macrophages regulate hyaluronan binding upon inflammatory and anti-inflammatory stimuli. Mouse bone marrow-derived macrophages stimulated with tumor necrosis factor α or lipopolysaccharide and interferon-γ (LPS/IFNγ) induced hyaluronan binding by up-regulating CD44 and down-regulating chondroitin sulfation on CD44. Hyaluronan binding was induced to a lesser extent in interleukin-4 (IL-4)-activated macrophages despite increased CD44 expression, and this was attributable to increased chondroitin sulfation on CD44, as treatment with β-d-xyloside to prevent chondroitin sulfate addition significantly enhanced hyaluronan binding. These changes in the chondroitin sulfation of CD44 were associated with changes in mRNA expression of two chondroitin sulfotransferases, CHST3 and CHST7, which were decreased in LPS/IFNγ-stimulated macrophages and increased in IL-4-stimulated macrophages. Thus, inflammatory and anti-inflammatory stimuli differentially regulate the chondroitin sulfation of CD44, which is a dynamic physiological regulator of hyaluronan binding by CD44 in mouse macrophages.
Figures
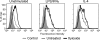
Similar articles
-
Analysis of CD44 interactions with hyaluronan in murine L cell fibroblasts deficient in glycosaminoglycan synthesis: a role for chondroitin sulfate.J Cell Sci. 1998 Apr;111 ( Pt 7):1021-9. doi: 10.1242/jcs.111.7.1021. J Cell Sci. 1998. PMID: 9490645
-
TNFalpha and IL-4 regulation of hyaluronan binding to monocyte CD44 involves posttranslational modification of CD44.Cell Immunol. 1999 May 1;193(2):209-18. doi: 10.1006/cimm.1999.1456. Cell Immunol. 1999. PMID: 10222064
-
Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14(+) peripheral blood monocytes.J Immunol. 2001 Nov 1;167(9):5367-74. doi: 10.4049/jimmunol.167.9.5367. J Immunol. 2001. PMID: 11673554
-
CD44 and its role in inflammation and inflammatory diseases.Inflamm Allergy Drug Targets. 2009 Jul;8(3):208-20. doi: 10.2174/187152809788680994. Inflamm Allergy Drug Targets. 2009. PMID: 19601881 Review.
-
A role for the cell adhesion molecule CD44 and sulfation in leukocyte-endothelial cell adhesion during an inflammatory response?Biochem Pharmacol. 2000 Mar 1;59(5):455-65. doi: 10.1016/s0006-2952(99)00266-x. Biochem Pharmacol. 2000. PMID: 10660111 Review.
Cited by
-
Regenerative potential of glycosaminoglycans for skin and bone.J Mol Med (Berl). 2012 Jun;90(6):625-35. doi: 10.1007/s00109-011-0843-2. Epub 2011 Dec 21. J Mol Med (Berl). 2012. PMID: 22187113 Review.
-
CD44 deficiency inhibits unloading-induced cortical bone loss through downregulation of osteoclast activity.Sci Rep. 2015 Nov 4;5:16124. doi: 10.1038/srep16124. Sci Rep. 2015. PMID: 26530337 Free PMC article.
-
Cell Therapy of Corneal Diseases.Cornea. 2016 Nov;35 Suppl 1(Suppl 1):S9-S19. doi: 10.1097/ICO.0000000000001010. Cornea. 2016. PMID: 27631350 Free PMC article. Review.
-
A Novel Splice Variant of HYAL-4 Drives Malignant Transformation and Predicts Outcome in Patients with Bladder Cancer.Clin Cancer Res. 2020 Jul 1;26(13):3455-3467. doi: 10.1158/1078-0432.CCR-19-2912. Epub 2020 Feb 24. Clin Cancer Res. 2020. PMID: 32094233 Free PMC article.
-
CD44-mediated hyaluronan binding marks proliferating hematopoietic progenitor cells and promotes bone marrow engraftment.PLoS One. 2018 Apr 23;13(4):e0196011. doi: 10.1371/journal.pone.0196011. eCollection 2018. PLoS One. 2018. PMID: 29684048 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

