A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus
- PMID: 21471243
- PMCID: PMC3126318
- DOI: 10.1128/JVI.00267-11
A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus
Abstract
Herpes simplex virus (HSV) entry requires host cell 26S proteasomal degradation activity at a postpenetration step. When expressed in the infected cell, the HSV immediate-early protein ICP0 has E3 ubiquitin ligase activity and interacts with the proteasome. The cell is first exposed to ICP0 during viral entry, since ICP0 is a component of the inner tegument layer of the virion. The function of tegument ICP0 is unknown. Deletion of ICP0 or mutations in the N-terminal RING finger domain of ICP0 results in the absence of ICP0 from the tegument. We show here that these mutations negatively influenced the targeting of incoming capsids to the nucleus. Inhibitors of the chymotrypsin-like activity of the proteasome the blocked entry of virions containing tegument ICP0, including ICP0 mutants that are defective in USP7 binding. However, ICP0-deficient virions were not blocked by proteasomal inhibitors and entered cells via a proteasome-independent mechanism. ICP0 appeared to play a postpenetration role in cells that supported either endocytosis or nonendosomal entry pathways for HSV. The results suggest that ICP0 mutant virions are defective upstream of viral gene expression at a pre-immediate-early step in infection. We propose that proteasome-mediated degradation of a virion or host protein is regulated by ICP0 to allow efficient delivery of entering HSV capsids to the nuclear periphery.
Figures

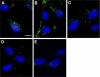




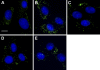
References
-
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. 1973. Genetic studies with herpes simplex virus type 1: the isolation of temperature-sensitive mutants, their arrangement into complementation groups, and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329–346 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

