Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells
- PMID: 21471245
- PMCID: PMC3126327
- DOI: 10.1128/JVI.00155-11
Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells
Abstract
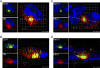
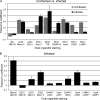
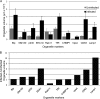
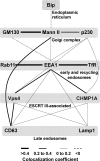
Human cytomegalovirus (HCMV) induces extensive remodeling of the secretory apparatus to form the cytoplasmic virion assembly compartment (cVAC), where virion tegumentation and envelopment take place. We studied the structure of the cVAC by confocal microscopy to assess the three-dimensional distribution of proteins specifically associated with individual secretory organelles. In infected cells, early endosome antigen 1 (EEA1)-positive vesicles are concentrated at the center of the cVAC and, as previously seen, are distinct from structures visualized by markers for the endoplasmic reticulum, Golgi apparatus, and trans-Golgi network (TGN). EEA1-positive vesicles can be strongly associated with markers for recycling endosomes, to a lesser extent with markers associated with components of the endosomal sorting complex required for transport III (ESCRT III) machinery, and then with markers of late endosomes. In comparisons of uninfected and infected cells, we found significant changes in the structural associations and colocalization of organelle markers, as well as in net organelle volumes. These results provide new evidence that the HCMV-induced remodeling of the membrane transport apparatus involves much more than simple relocation and expansion of preexisting structures and are consistent with the hypothesis that the shift in identity of secretory organelles in HCMV-infected cells results in new functional profiles.
Figures




References
-
- Barr F. A., Short B. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405–413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

