Mild cognitive impairment: baseline and longitudinal structural MR imaging measures improve predictive prognosis
- PMID: 21471273
- PMCID: PMC3099042
- DOI: 10.1148/radiol.11101975
Mild cognitive impairment: baseline and longitudinal structural MR imaging measures improve predictive prognosis
Abstract
Purpose: To assess whether single-time-point and longitudinal volumetric magnetic resonance (MR) imaging measures provide predictive prognostic information in patients with amnestic mild cognitive impairment (MCI).
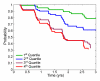
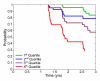
Materials and methods: This study was conducted with institutional review board approval and in compliance with HIPAA regulations. Written informed consent was obtained from all participants or the participants' legal guardians. Cross-validated discriminant analyses of MR imaging measures were performed to differentiate 164 Alzheimer disease (AD) cases from 203 healthy control cases. Separate analyses were performed by using data from MR images obtained at one time point or by combining single-time-point measures with 1-year change measures. Resulting discriminant functions were applied to 317 MCI cases to derive individual patient risk scores. Risk of conversion to AD was estimated as a continuous function of risk score percentile. Kaplan-Meier survival curves were computed for risk score quartiles. Odds ratios (ORs) for the conversion to AD were computed between the highest and lowest quartile scores.
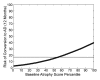
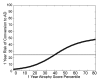
Results: Individualized risk estimates from baseline MR examinations indicated that the 1-year risk of conversion to AD ranged from 3% to 40% (average group risk, 17%; OR, 7.2 for highest vs lowest score quartiles). Including measures of 1-year change in global and regional volumes significantly improved risk estimates (P = 001), with the risk of conversion to AD in the subsequent year ranging from 3% to 69% (average group risk, 27%; OR, 12.0 for highest vs lowest score quartiles).
Conclusion: Relative to the risk of conversion to AD conferred by the clinical diagnosis of MCI alone, MR imaging measures yield substantially more informative patient-specific risk estimates. Such predictive prognostic information will be critical if disease-modifying therapies become available.
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11101975/-/DC1.
RSNA, 2011
Figures

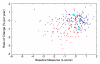
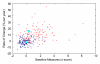
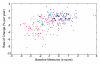
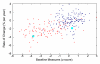

References
-
- Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology 2007;69(16):1622–1634 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

