Overexpression of mitotic centromere-associated Kinesin stimulates microtubule detachment and confers resistance to paclitaxel
- PMID: 21471284
- PMCID: PMC3112244
- DOI: 10.1158/1535-7163.MCT-10-1109
Overexpression of mitotic centromere-associated Kinesin stimulates microtubule detachment and confers resistance to paclitaxel
Abstract
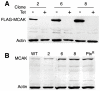
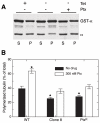
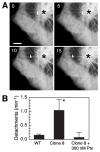
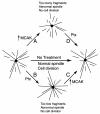
Numerous studies have implicated mutations in tubulin or the overexpression of specific tubulin genes in resistance to microtubule-targeted drugs. Much less is known about the role of accessory proteins that modulate microtubule behavior in the genesis of drug resistance. Here, we examine mitotic centromere-associated kinesin (MCAK), a member of the kinesin family of microtubule motor proteins that has the ability to stimulate microtubule depolymerization, and show that overexpressing the protein confers resistance to paclitaxel and epothilone A, but increases sensitivity to colcemid. Cells transfected with FLAG-tagged MCAK cDNA using a tet-off-regulated expression system had a disrupted microtubule cytoskeleton and were able to survive a toxic concentration of paclitaxel in the absence, but not in the presence of tetracycline, showing that drug resistance was caused by ectopic MCAK production. Moreover, a population that was heterogeneous with respect to FLAG-MCAK expression became enriched with cells that produced the ectopic protein when it was placed under paclitaxel selection. Similar to previously isolated mutants with altered tubulin, paclitaxel resistant cells resulting from MCAK overexpression were found to have decreased microtubule polymer and a seven-fold increase in the frequency of microtubule detachment from centrosomes. These data are consistent with a model for paclitaxel resistance that is based on stability of the attachment of microtubules to their nucleating centers, and they implicate MCAK in the mechanism of microtubule detachment.
Figures



References
-
- Cabral F. Factors determining cellular mechanisms of resistance to antimitotic drugs. Drug Resistance Updates. 2001;3:1–6. - PubMed
-
- Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–9. - PubMed
-
- Ogawa T, Nitta R, Okada Y, Hirokawa N. A common mechanism for microtubule destabilizers-M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell. 2004;116:591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

