Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts
- PMID: 21471287
- PMCID: PMC3096691
- DOI: 10.1158/1541-7786.MCR-10-0565
Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts
Abstract
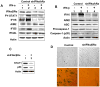
The IFN-inducible IFI16 and AIM2 proteins act as innate immune sensors for cytosolic double-stranded DNA (dsDNA). On sensing dsDNA, the IFI16 protein induces the expression of IFN-β whereas the AIM2 protein forms an inflammasome, which promotes the secretion of IL-1β. Given that the knockdown of IFI16 expression in human diploid fibroblasts (HDF) delays the onset of cellular senescence, we investigated the potential roles for the IFI16 and AIM2 proteins in cellular senescence. We found that increased IFI16 protein levels in old (vs. young) HDFs were associated with the induction of IFN-β. In contrast, increased levels of the AIM2 protein in the senescent (vs. old) HDFs were associated with increased production of IL-1β. The knockdown of type I IFN-α receptor subunit, which reduced the basal levels of the IFI16 but not of the AIM2, protein delayed the onset of cellular senescence. Accordingly, increased constitutive levels of IFI16 and AIM2 proteins in ataxia telangiectasia mutated (ATM) HDFs were associated with the activation of the IFN signaling and increased levels of IL-1β. The IFN-β treatment of the young HDFs, which induced the expression of IFI16 and AIM2 proteins, activated a DNA damage response and also increased basal levels of IL-1β. Interestingly, the knockdown of AIM2 expression in HDFs increased the basal levels of IFI16 protein and activated the IFN signaling. In contrast, the knockdown of the IFI16 expression in HDFs decreased the basal and dsDNA-induced activation of the IFN signaling. Collectively, our observations show differential roles for the IFI16 and AIM2 proteins in cellular senescence and associated secretory phenotype.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

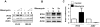






References
-
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27– 31. - PubMed
-
- Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–49. - PubMed
-
- Mammone T, Gan D, Foyouzi-Youssefi R. Apoptotic cell death increases with senescence in normal human dermal fibroblast cultures. Cell Biol Int. 2006;30:903–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

