The basis for diminished functional recovery after delayed peripheral nerve repair
- PMID: 21471367
- PMCID: PMC6622714
- DOI: 10.1523/JNEUROSCI.6156-10.2011
The basis for diminished functional recovery after delayed peripheral nerve repair
Abstract
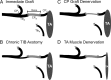
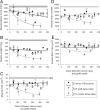
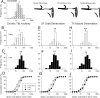
The postsurgical period during which neurons remain without target connections (chronic axotomy) and distal nerve stumps and target muscles are denervated (chronic denervation) deleteriously affects functional recovery. An autologous nerve graft and cross-suture paradigm in Sprague Dawley rats was used to systematically and independently control time of motoneuron axotomy, denervation of distal nerve sheaths, and muscle denervation to determine relative contributions of each factor to recovery failure. Tibial (TIB) nerve was cross-sutured to common peroneal (CP) nerve via a contralateral 15 mm nerve autograft to reinnervate the tibialis anterior (TA) muscle immediately or after prolonging TIB axotomy, CP autograft denervation, or TA muscle denervation. Numbers of motoneurons that reinnervated TA muscle declined exponentially from 99 ± 15 to asymptotic mean (± SE) values of 35 ± 1, 41 ± 10, and 13 ± 5, respectively. Enlarged reinnervated motor units fully compensated for reduced motoneuron numbers after prolonged axotomy and autograft denervation, but the maximal threefold enlargement did not compensate for the severe loss of regenerating nerves through chronically denervated nerve stumps and for failure of reinnervated muscle fibers to recover from denervation atrophy. Muscle force, weight, and cross-sectional area declined. Our results demonstrate that chronic denervation of the distal stump plays a key role in reduced nerve regeneration, but the denervated muscle is also a contributing factor. That chronic Schwann cell denervation within the nerve autograft reduced regeneration less than after the denervation of both CP nerve stump and TA muscle, argues that chronic muscle denervation negatively impacts nerve regeneration.
Figures



References
-
- Anzil AP, Wernig A. Muscle fibre loss and reinnervation after long-term denervation. J Neurocytol. 1989;18:833–845. - PubMed
-
- Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–510. - PubMed
-
- Befort K, Karchewski L, Lanoue C, Woolf CJ. Selective up-regulation of the growth arrest DNA damage-inducible gene Gadd45 alpha in sensory and motor neurons after peripheral nerve injury. Eur J Neurosci. 2003;18:911–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
