Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias
- PMID: 21471469
- PMCID: PMC3100134
- DOI: 10.1212/WNL.0b013e31821a4439
Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias
Abstract
Background: Diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) MRI have high sensitivity and specificity for Creutzfeldt-Jakob disease (CJD). No studies, however, have demonstrated how MRI can distinguish CJD from nonprion causes of rapidly progressive dementia (npRPD). We sought to determine the diagnostic accuracy of MRI for CJD compared to a cohort of npRPD subjects.
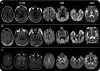
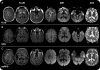
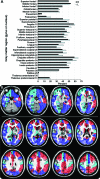
Methods: Two neuroradiologists blinded to diagnosis assessed DWI and FLAIR images in 90 patients with npRPD (n = 29) or prion disease (sporadic CJD [sCJD], n = 48, or genetic prion disease [familial CJD, n = 6, and Gerstmann-Sträussler-Scheinker, n = 7]). Thirty-one gray matter regions per hemisphere were assessed for abnormal hyperintensities. The likelihood of CJD was assessed using our previously published criteria.
Results: Gray matter hyperintensities (DWI > FLAIR) were found in all sCJD cases, with certain regions preferentially involved, but never only in limbic regions, and rarely in the precentral gyrus. In all sCJD cases with basal ganglia or thalamic DWI hyperintensities, there was associated restricted diffusion (apparent diffusion coefficient [ADC] map). This restricted diffusion, however, was not seen in any npRPD cases, in whom isolated limbic hyperintensities (FLAIR > DWI) were common. One reader's sensitivity and specificity for sCJD was 94% and 100%, respectively, the other's was 92% and 72%. After consensus review, the readers' combined MRI sensitivity and specificity for sCJD was 96% and 93%, respectively. Familial CJD had overlapping MRI features with sCJD.
Conclusions: The pattern of FLAIR/DWI hyperintensity and restricted diffusion can differentiate sCJD from other RPDs with a high sensitivity and specificity. MRI with DWI and ADC should be included in sCJD diagnostic criteria. New sCJD MRI criteria are proposed.
Figures
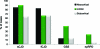
References
-
- Shiga Y, Miyazawa K, Sato S, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology 2004;I63:443–449 - PubMed
-
- Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol 1996;53:913–920 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
