The antifibrinolytic function of factor XIII is exclusively expressed through α₂-antiplasmin cross-linking
- PMID: 21471521
- PMCID: PMC3448560
- DOI: 10.1182/blood-2011-02-333203
The antifibrinolytic function of factor XIII is exclusively expressed through α₂-antiplasmin cross-linking
Erratum in
- Blood. 2011 Dec 22;118(26):6993
Abstract
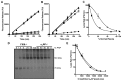
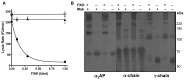
Factor XIII (FXIII) generates fibrin-fibrin and fibrin-inhibitor cross-links. Our flow model, which is sensitive to cross-linking, was used to assess the effects of FXIII and the fibrinolytic inhibitor, α₂-antiplasmin (α₂AP) on fibrinolysis. Plasma model thrombi formed from FXIII or α₂AP depleted plasma lysed at strikingly similar rates, 9-fold faster than pooled normal plasma (PNP). In contrast, no change was observed on depletion of PAI-1 or thrombin activatable fibrinolysis inhibitor (TAFI). Inhibition of FXIII did not further enhance lysis of α₂AP depleted thrombi. Addition of PNP to FXIII or α₂AP depleted plasmas normalized lysis. Lysis rate was strongly inversely correlated with total cross-linked α₂AP in plasma thrombi. Reconstitution of FXIII into depleted plasma stabilized plasma thrombi and normalized γ-dimers and α-polymers formation. However, the presence of a neutralizing antibody to α₂AP abolished this stabilization. Our data show that the antifibrinolytic function of FXIII is independent of fibrin-fibrin cross-linking and is expressed exclusively through α₂AP.
Figures
Similar articles
-
Inhibition of Fibrinolysis by Coagulation Factor XIII.Biomed Res Int. 2017;2017:1209676. doi: 10.1155/2017/1209676. Epub 2017 Jul 6. Biomed Res Int. 2017. PMID: 28761875 Free PMC article. Review.
-
TAFIa, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots.J Thromb Haemost. 2007 Apr;5(4):812-7. doi: 10.1111/j.1538-7836.2007.02430.x. Epub 2007 Feb 2. J Thromb Haemost. 2007. PMID: 17388801
-
Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII.J Thromb Haemost. 2016 Jul;14(7):1453-61. doi: 10.1111/jth.13354. Epub 2016 Jun 9. J Thromb Haemost. 2016. PMID: 27148673
-
Functional factor XIII-A is exposed on the stimulated platelet surface.Blood. 2014 Dec 18;124(26):3982-90. doi: 10.1182/blood-2014-06-583070. Epub 2014 Oct 20. Blood. 2014. PMID: 25331118 Free PMC article.
-
Novel aspects of blood coagulation factor XIII. I. Structure, distribution, activation, and function.Crit Rev Clin Lab Sci. 1996;33(5):357-421. doi: 10.3109/10408369609084691. Crit Rev Clin Lab Sci. 1996. PMID: 8922891 Review.
Cited by
-
Incorporation of albumin fusion proteins into fibrin clots in vitro and in vivo: comparison of different fusion motifs recognized by factor XIIIa.BMC Biotechnol. 2011 Dec 20;11:127. doi: 10.1186/1472-6750-11-127. BMC Biotechnol. 2011. PMID: 22185689 Free PMC article.
-
Control of fibrinolytic drug injection via real-time ultrasonic monitoring of blood coagulation.PLoS One. 2019 Feb 27;14(2):e0211646. doi: 10.1371/journal.pone.0211646. eCollection 2019. PLoS One. 2019. PMID: 30811424 Free PMC article.
-
Factor XIII cotreatment with hemostatic agents in hemophilia A increases fibrin α-chain crosslinking.J Thromb Haemost. 2018 Jan;16(1):131-141. doi: 10.1111/jth.13887. Epub 2017 Nov 20. J Thromb Haemost. 2018. PMID: 29080382 Free PMC article.
-
Inhibition of Fibrinolysis by Coagulation Factor XIII.Biomed Res Int. 2017;2017:1209676. doi: 10.1155/2017/1209676. Epub 2017 Jul 6. Biomed Res Int. 2017. PMID: 28761875 Free PMC article. Review.
-
The Efficacy of Fibrinogen Concentrates in Relation to Cryoprecipitate in Restoring Clot Integrity and Stability against Lysis.Int J Mol Sci. 2022 Mar 9;23(6):2944. doi: 10.3390/ijms23062944. Int J Mol Sci. 2022. PMID: 35328366 Free PMC article.
References
-
- Mockros LF, Roberts WW, Lorand L. Viscoelastic properties of ligation-inhibited fibrin clots. Biophys Chem. 1974;2(2):164–169. - PubMed
-
- Anwar R, Miloszewski KJ. Factor XIII deficiency. Br J Haematol. 1999;107(3):468–484. - PubMed
-
- Koseki-Kuno S, Yamakawa M, Dickneite G, Ichinose A. Factor XIII A subunit-deficient mice developed severe uterine bleeding events and subsequent spontaneous miscarriages. Blood. 2003;102(13):4410–4412. - PubMed
-
- Chen R, Doolittle RF. - cross-linking sites in human and bovine fibrin. Biochemistry. 1971;10(24):4487–4491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

