Assessment of the in vivo genotoxicity of new lead compounds to treat sickle cell disease
- PMID: 21471937
- PMCID: PMC6260610
- DOI: 10.3390/molecules16042982
Assessment of the in vivo genotoxicity of new lead compounds to treat sickle cell disease
Abstract
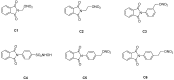
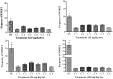
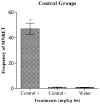
The compounds 1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl nitrate (C1), (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethyl nitrate (C2), 3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)benzyl nitrate (C3), 4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-N-hydroxy-benzenesulfonamide (C4), 4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)benzyl nitrate (C5), and 2-[4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)phenyl]ethyl nitrate (C6) were evaluated with a micronucleus test using mouse peripheral blood to identify new candidate drugs for the treatment of sickle cell disease (SCD) that are safer than hydroxyurea. The compounds induced an average frequency of micronucleated reticulocytes (MNRET) of less than six per 1,000 cells at 12.5, 25, 50, and 100 mg/kg, whereas hydroxyurea induced an average MNRET frequency of 7.8, 9.8, 15, and 33.7 per 1000 cells respectively, at the same concentrations. Compounds C1-C6 are new non-genotoxic in vivo candidate drugs for the treatment of SCD symptoms.
Figures
References
-
- Hanft V.N., Fruchtman S.R., Pickens C.V., Rosse W.F., Howard T.A., Ware R.E. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood. 2000;95:3589–3593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

