BAG3: a multifaceted protein that regulates major cell pathways
- PMID: 21472004
- PMCID: PMC3122056
- DOI: 10.1038/cddis.2011.24
BAG3: a multifaceted protein that regulates major cell pathways
Abstract
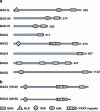
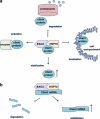
Bcl2-associated athanogene 3 (BAG3) protein is a member of BAG family of co-chaperones that interacts with the ATPase domain of the heat shock protein (Hsp) 70 through BAG domain (110-124 amino acids). BAG3 is the only member of the family to be induced by stressful stimuli, mainly through the activity of heat shock factor 1 on bag3 gene promoter. In addition to the BAG domain, BAG3 contains also a WW domain and a proline-rich (PXXP) repeat, that mediate binding to partners different from Hsp70. These multifaceted interactions underlie BAG3 ability to modulate major biological processes, that is, apoptosis, development, cytoskeleton organization and autophagy, thereby mediating cell adaptive responses to stressful stimuli. In normal cells, BAG3 is constitutively present in a very few cell types, including cardiomyocytes and skeletal muscle cells, in which the protein appears to contribute to cell resistance to mechanical stress. A growing body of evidence indicate that BAG3 is instead expressed in several tumor types. In different tumor contexts, BAG3 protein was reported to sustain cell survival, resistance to therapy, and/or motility and metastatization. In some tumor types, down-modulation of BAG3 levels was shown, as a proof-of-principle, to inhibit neoplastic cell growth in animal models. This review attempts to outline the emerging mechanisms that can underlie some of the biological activities of the protein, focusing on implications in tumor progression.
Figures
Similar articles
-
Role of BAG3 in cancer progression: A therapeutic opportunity.Semin Cell Dev Biol. 2018 Jun;78:85-92. doi: 10.1016/j.semcdb.2017.08.049. Epub 2017 Aug 31. Semin Cell Dev Biol. 2018. PMID: 28864347 Review.
-
Bcl2-associated athanogene 3 interactome analysis reveals a new role in modulating proteasome activity.Mol Cell Proteomics. 2013 Oct;12(10):2804-19. doi: 10.1074/mcp.M112.025882. Epub 2013 Jul 3. Mol Cell Proteomics. 2013. PMID: 23824909 Free PMC article.
-
Unraveling the mystery: How bad is BAG3 in hematological malignancies?Biochim Biophys Acta Rev Cancer. 2022 Sep;1877(5):188781. doi: 10.1016/j.bbcan.2022.188781. Epub 2022 Aug 17. Biochim Biophys Acta Rev Cancer. 2022. PMID: 35985611 Review.
-
MicroRNA regulation of BAG3.Exp Biol Med (Maywood). 2022 Apr;247(8):617-623. doi: 10.1177/15353702211066908. Epub 2022 Jan 15. Exp Biol Med (Maywood). 2022. PMID: 35037515 Free PMC article. Review.
-
Corelating the molecular structure of BAG3 to its oncogenic role.Cell Biol Int. 2024 Aug;48(8):1080-1096. doi: 10.1002/cbin.12199. Epub 2024 Jun 23. Cell Biol Int. 2024. PMID: 38924608 Review.
Cited by
-
A weakened interface in the P182L variant of HSP27 associated with severe Charcot-Marie-Tooth neuropathy causes aberrant binding to interacting proteins.EMBO J. 2021 Apr 15;40(8):e103811. doi: 10.15252/embj.2019103811. Epub 2021 Mar 1. EMBO J. 2021. PMID: 33644875 Free PMC article.
-
Human coronaviruses activate and hijack the host transcription factor HSF1 to enhance viral replication.Cell Mol Life Sci. 2024 Sep 7;81(1):386. doi: 10.1007/s00018-024-05370-5. Cell Mol Life Sci. 2024. PMID: 39243335 Free PMC article.
-
The prosurvival protein BAG3: a new participant in vascular homeostasis.Cell Death Dis. 2016 Oct 20;7(10):e2431. doi: 10.1038/cddis.2016.321. Cell Death Dis. 2016. PMID: 27763645 Free PMC article.
-
Nanomaterial-mediated low-temperature photothermal therapy via heat shock protein inhibition.Front Bioeng Biotechnol. 2022 Oct 11;10:1027468. doi: 10.3389/fbioe.2022.1027468. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36304896 Free PMC article. Review.
-
Structural Refinement of 2,4-Thiazolidinedione Derivatives as New Anticancer Agents Able to Modulate the BAG3 Protein.Molecules. 2022 Jan 20;27(3):665. doi: 10.3390/molecules27030665. Molecules. 2022. PMID: 35163936 Free PMC article.
References
-
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. - PubMed
-
- Rosati A, Ammirante M, Gentilella A, Basile A, Festa M, Pascale M, et al. Apoptosis inhibition in cancer cells: a novel molecular pathway that involves BAG3 protein. Int J Biochem Cell Biol. 2007;39:1337–1342. - PubMed
-
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:237–241. - PubMed
-
- Doukhanina EV, Chen S, van der Zalm E, Godzik A, Reed J, Dickman MB. Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem. 2006;281:18793–18801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

