Optimization of circulating biomarkers of obatoclax-induced cell death in patients with small cell lung cancer
- PMID: 21472138
- PMCID: PMC3071082
- DOI: 10.1593/neo.101524
Optimization of circulating biomarkers of obatoclax-induced cell death in patients with small cell lung cancer
Abstract
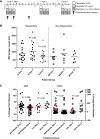
Small cell lung cancer (SCLC) is an aggressive disease in which, after initial sensitivity to platinum/etoposide chemotherapy, patients frequently relapse with drug-resistant disease. Deregulation of the Bcl-2 pathway is implicated in the pathogenesis of SCLC, and early phase studies of Bcl-2 inhibitors have been initiated in SCLC. Obatoclax is a small-molecule drug designed to target the antiapoptotic Bcl-2 family members to a proapoptotic effect. Preclinical studies were conducted to clarify the kinetics of obatoclax-induced apoptosis in a panel of SCLC cell lines to assist with the interpretation of biomarker data generated during early phase clinical trials. In vitro, obatoclax was synergistic with cisplatin and etoposide, and "priming" cells with obatoclax before the cytotoxics maximized tumor cell death. Peak levels of apoptosis, reflected by cleaved cytokeratin 18 (CK18) levels (M30 ELISA) and caspase activity (SR-DEVD-FMK), occurred 24 hours after obatoclax treatment. A phase 1b-2 trial of obatoclax administered using two infusion regimens in combination with carboplatin and etoposide has been completed in previously untreated patients with extensive-stage SCLC. Circulating pharmacodynamic biomarkers of cell death, full-length and/or cleaved CK18, and oligonucleosomal DNA were studied in the phase 1b trial. All SCLC patients classified as "responders" after two cycles of treatment showed significantly increased levels of full-length and cleaved CK18 (M65 ELISA) on day 3 of study. However, the preclinical data and the absence of a peak in circulating caspase-cleaved CK18 in trial patients suggest suboptimal timing of blood sampling, which will need refinement in future trials incorporating obatoclax.
Figures
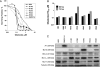
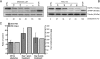
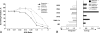
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R, Boye N, Wang M, Vigander T, Vilsvik J, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20(24):4665–4672. - PubMed
-
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8(2):121–132. - PubMed
-
- Buolamwini JK. Novel anticancer drug discovery. Curr Opin Chem Biol. 1999;3(4):500–509. - PubMed
-
- Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of Bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54(1):6–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
