Anion-dependent fluorescence in bis(anilinoethynyl)pyridine derivatives: switchable ON-OFF and OFF-ON responses
- PMID: 21472189
- PMCID: PMC4028034
- DOI: 10.1039/c1cc10947b
Anion-dependent fluorescence in bis(anilinoethynyl)pyridine derivatives: switchable ON-OFF and OFF-ON responses
Abstract
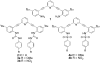
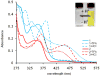
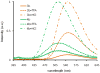
Urea and sulfonamide derivatives of 1 exhibit ON-OFF and OFF-ON switchable fluorescent and colorimetric responses upon protonation. The magnitude of the fluorescence event is dictated by the anion, resulting in a rare, fully organic "turn-on" fluorescent sensor for chloride.
© The Royal Society of Chemistry 2011
Figures



Similar articles
-
"Off-on" aggregation-based fluorescent sensor for the detection of chloride in water.Org Biomol Chem. 2015 Apr 14;13(14):4266-70. doi: 10.1039/c4ob02409e. Epub 2015 Mar 11. Org Biomol Chem. 2015. PMID: 25758666 Free PMC article.
-
A pyridinium cation-π interaction sensor for the fluorescent detection of alkyl halides.Chem Commun (Camb). 2011 Jan 7;47(1):253-5. doi: 10.1039/c0cc01420f. Epub 2010 Jul 5. Chem Commun (Camb). 2011. PMID: 20601994
-
Selective detection of NADPH among four pyridine-nucleotide cofactors by a fluorescent probe based on aggregation-induced emission.Macromol Rapid Commun. 2013 May 14;34(9):779-84. doi: 10.1002/marc.201300015. Epub 2013 Mar 13. Macromol Rapid Commun. 2013. PMID: 23495077
-
Rigidization, preorientation and electronic decoupling--the 'magic triangle' for the design of highly efficient fluorescent sensors and switches.Chem Soc Rev. 2002 Mar;31(2):116-27. doi: 10.1039/b100604p. Chem Soc Rev. 2002. PMID: 12109205 Review.
-
Fluorescent analogs of biomolecular building blocks: design, properties, and applications.Chem Rev. 2010 May 12;110(5):2579-619. doi: 10.1021/cr900301e. Chem Rev. 2010. PMID: 20205430 Free PMC article. Review. No abstract available.
Cited by
-
Intensified electrochemiluminescence and photoluminescence via supramolecular anion recognition interactions.Chem Sci. 2024 Jul 4;15(31):12291-12300. doi: 10.1039/d4sc03338h. eCollection 2024 Aug 7. Chem Sci. 2024. PMID: 39118623 Free PMC article.
-
Harnessing solid-state packing for selective detection of chloride in a macrocyclic anionophore.Chem Commun (Camb). 2016 Jul 21;52(61):9506-9. doi: 10.1039/c6cc03795j. Chem Commun (Camb). 2016. PMID: 27375117 Free PMC article.
-
Solid-State Examination of Conformationally Diverse Sulfonamide Receptors Based on Bis(2-anilinoethynyl)pyridine, -Bipyridine, and -Thiophene.Cryst Growth Des. 2015 Mar 4;15(3):1502-1511. doi: 10.1021/cg5018856. Cryst Growth Des. 2015. PMID: 26405435 Free PMC article.
-
Disrupting the Hofmeister bias in salt liquid-liquid extraction with an arylethynyl bisurea anion receptor.Chem Sci. 2024 Mar 7;15(14):5311-5318. doi: 10.1039/d3sc05922g. eCollection 2024 Apr 3. Chem Sci. 2024. PMID: 38577371 Free PMC article.
-
Spermine detection via metal-mediated ethynylarene 'turn-on' fluorescence signaling.Sens Actuators B Chem. 2015 Feb 1;207(Pt A):843-848. doi: 10.1016/j.snb.2014.10.116. Sens Actuators B Chem. 2015. PMID: 25530671 Free PMC article.
References
-
-
For reviews of small molecule biological imaging see: Kikuchi K. Chem Soc Rev. 2010;39:2048.Carroll CN, Naleway J, Haley MM, Johnson DW. Chem Soc Rev. 2010;39:3875.
-
-
-
For reviews of molecular logic see: Mirkin CA, Ratner MA. Molecular Electronics. Vol. 43 Annual Reviews, Inc.; 1992. Feringa BL. Molecular Switches. Wiley-VCH GmbH; Weinheim, Germany: 2001. Montalban AG, Baum SM, Barrett AGM, Hoffman BM. Dalton Trans. 2003;2093Norum OJ, Selbo PK, Weyergang A, Giercksky KE, Berg K. J Photochem Photobiol B. 2009;96:83.
-
-
-
(a) Themed issue in supramolecular chemistry of anions and references therein: Gale PA, Gunnlaugsson T. Chem Soc Rev. 2010;39:3595.
- Valeur B. Molecular Fluorescence. Vol. 273 Wiley-VCH Verlag GmbH; Weinheim, Germany: 2002.
-
-
- Fabbrizzi L, Gatti F, Pallavicini P, Parodi L. New J Chem. 1998;1403
- Shizuka H, Tobita S. J Am Chem Soc. 1982;104:6919.
- Clements JH, Webber SE. Macromolecules. 2004;37:1531.
- de Silva AP, Nimal Gunaratne HQ, McCoy CP. Chem Commun. 1996:2399.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

